Team:Heidelberg/Notebook/Killing II/12thweek
From 2008.igem.org
(Difference between revisions)
(→Killer-prey system test and lysis test of killer cells) |
(→Characterization: ColicinE1-Receiver Activitytest: Killer-prey system test and lysis test of killer cells) |
||
| Line 609: | Line 609: | ||
*8.30 pm: Preparing mixtures for the plate: | *8.30 pm: Preparing mixtures for the plate: | ||
| - | + | **for colE1-Receiver killer-prey test (left part of the plate): | |
| + | ** 1:4 100 µl Sender + 400 µl Receivercells | ||
| + | ** 1:1 100 µl Sender + 100 µl Receivercells + 300 µl TB media | ||
| + | ** 5:1 100 µl Sender + 20 µl Receivercells + 380 µl TB media | ||
| + | ** 10:1 100 µl Sender + 10 µl Receivercells + 390 µl TB media | ||
| + | ** 25:1 100 µl Sender + 4 µl Receivercells + 396 µl TB media | ||
| + | ** 50:1 100 µl Sender + 2 µl Receivercells + 398 µl TB media | ||
| + | **100:1 100 µl Sender + 0.4 µl Receivercells + 400 µl TB media | ||
| + | **for colE1-Receiver lysis test (right part of the plate) | ||
| + | **colE1 + I20260: 250 µl colE1 cells + 250 µl TB-Amp-Kana | ||
| + | **T9002 without GFP + I20260: 250 µl T9002 without GFP + I20260 cells + 250 µl TB-Amp-Kana | ||
| - | + | *plate scheme: | |
[[Image: 081025_killer_prey_lysis.jpg | 750 px | center]] | [[Image: 081025_killer_prey_lysis.jpg | 750 px | center]] | ||
Revision as of 11:49, 28 October 2008


12th week
Contents |
Monday 10/20/2008
pSB1A3-Receiver-Colicin cloning
- Minipreps of colE1/E9/E9lys-Receiver with QiaCube, Qiagen
- Send probes to GATC for sequencing
Sender cloning: constitutive promotor-sender
- Minipreps of J23107-Receiver with QiaCube, Qiagen
- Send probe to GATC for sequencing
Tuesday 10/21/2008
Sequencing results of ready cloned BioBrick parts
| EcoRI-site mutation | PstI-site 1 mutation | PstI-site 2 mutation | PstI-site 3 mutation | Prefix | Suffix | complete sequence | |
|---|---|---|---|---|---|---|---|
| colE1_BB_57-1 | + | + | + | + | + | + | + |
| colE1_BB_57-2 | + | + | + | + | + | + | + |
| colE1_BB_57+2 | ? | + | + | ? | + | ? | missing sequence |
| colE1_BB_67+1 | + | + | + | + | + | ? | sequencing failure |
| colE9_BB_(2) | + | XXX | XXX | XXX | + | + | + |
| colE9lys_BB | XXX | XXX | XXX | XXX | + | + | + |
| sender_BB | XXX | XXX | XXX | XXX | + | + | + |
--> colE1_BB_57-1, colE9_BB_(2), colE9lys_BB and sender_BB gave positive sequencing results in all criteria and will be sent to the registry
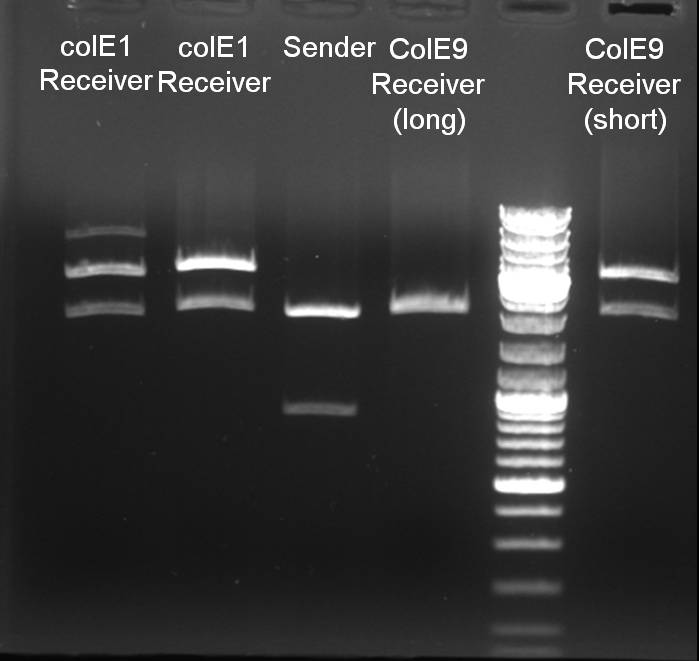
Controldigestion of parts with EcoRI and PstI
- colE1_BB_57-1, colE9_BB_(2), colE9lys_BB and sender_BB was digested with EcoRI and PstI: 1h 30 min -> 37 °C
0.5 µl EcoRI (NEB) 0.5 µl PstI (NEB) 3.0 µl DNA 2.0 µl EcoRI Buffer (NEB) 2.0 µl BSA 10x 12.0 µl H2O ------- 20.0 µl
- Gelresults: The digestion pattern looks like expected. 1% Agarose, 135 V, 30 min
Control of antibiotics resistance
- LB-media containing tetracycline, chloramphenicol, ampicilin or kanamycin was inoculated with the different parts to the theri antibiotics resistance.
Wednesday 10/22/2008
- Antibiotics test: Each part only grew in ampicilin media as expected
Thursday 10/23/2008
pSB1A2-Receiver-Colicin cloning
HisTag cloning of Colicins for purification
Colicin activity test
Friday 10/24/2008
pSB1A3-Receiver-Colicin cloning
HisTag cloning of Colicins for purification
Sender Cloning: pBAD - sender
Sender Cloning: constitutive promotor - sender
Saturday 10/25/2008
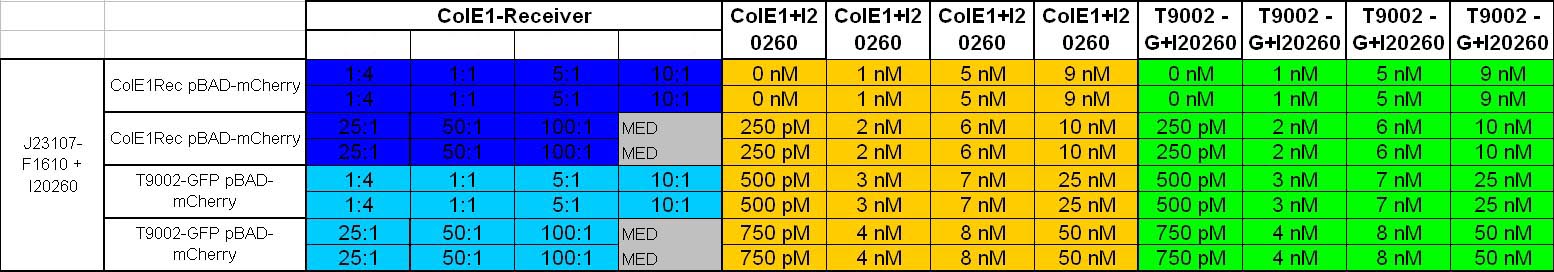
Characterization: ColicinE1-Receiver Activitytest: Killer-prey system test and lysis test of killer cells
- afternoon: Inoculation of the following cultures:
- constitutive Sender with GFP(J23107-F1610 + I20260)(TB-Kana_Amp)
- ColE1Rec pBAD-mCherry (TB-Kana-Amp-Arab)
- T9002 without GFP pBAD-mCherry (TB-Kana-Amp-Arab)
- ColE1Rec + I20260 (TB-Kana-Amp)
- T9002 without GFP + I20260 (TB-Kana-Amp)
- 8.30 pm: Preparing mixtures for the plate:
- for colE1-Receiver killer-prey test (left part of the plate):
- 1:4 100 µl Sender + 400 µl Receivercells
- 1:1 100 µl Sender + 100 µl Receivercells + 300 µl TB media
- 5:1 100 µl Sender + 20 µl Receivercells + 380 µl TB media
- 10:1 100 µl Sender + 10 µl Receivercells + 390 µl TB media
- 25:1 100 µl Sender + 4 µl Receivercells + 396 µl TB media
- 50:1 100 µl Sender + 2 µl Receivercells + 398 µl TB media
- 100:1 100 µl Sender + 0.4 µl Receivercells + 400 µl TB media
- for colE1-Receiver lysis test (right part of the plate)
- colE1 + I20260: 250 µl colE1 cells + 250 µl TB-Amp-Kana
- T9002 without GFP + I20260: 250 µl T9002 without GFP + I20260 cells + 250 µl TB-Amp-Kana
- plate scheme:
Theoretical work for documentation
Sunday 10/26/2008
Theoretical work for documentation.
 "
"