Team:Johns Hopkins/Notebook
From 2008.igem.org
Contents |
Groups
iGEM Groups 1.0
iGEM Groups 2.01
iGEM Groups 2.02
Important reminders and notes
[Make general comments here, so they don't get lost in peoples e-mail boxes]
July 11: Primers for group 1 were delivered yesterday.
July 11: Lab meeting at 7:30PM in the lab to go over miniprep protocol.
July 15: Lab meeting at 6:30PM with Jessica. Have status reports ready.
Bring labtop if you can.
July 17: Restriction Digest/Sequencing Preparation (with James) 6:00PM.
July 21: 6:00PM or 6:30PM Lab meeting with Jessica. Have status reports ready.
July 29: 7:00PM Lab Meeting. Meet in Conference Room across from lab. Have Status Reports ready
Aug. 05: 7:00PM Lab Meeting. Meet in Conference Room across from lab. Have Status Reports ready.
Aug. 12: 7:00PM Lab Meeting. Have Status Reports Ready.
Journal Club Topic: Fluorescent Proteins; James and Ingrid.
Aug. 19: 7:00PM Lab Meeting. Have Status Reports Ready.
Journal Club Topic: Yeast Mating Pathway/MAP Kinase Pathway ; Jasper and Tejas
Aug. 26: 7:00PM Lab Meeting. Have Status Reports Ready!
Journal Club Topic: S. cerevisiae Promoters; Allison and Nate
EVERY TUESDAY 7 PM LAB MEETING! Mudd 120
Journal Club
8/12/08
Fluorescent Proteins
Ingrid and James
Fluorescent Protein Powerpoint
Paper: Green Fluorescent Protein as a Marker for Gene Expression
8/19/08
Yeast Mating Pathway/MAP Kinase Pathway
Jasper and Tejas
MAP Kinase Powerpoint
Paper: Map Kinase Scaffold
8/19/08
Yeast Promoters
Allison and Nate
Gene structure powerpoint
Paper:
Genomic Footprinting of the Promoter Regions of STE2 and STE3 genes in the Yeast Saccharomyces cerevisiae
Additional Reading:
Interspecies variation reveals a conserved repressor of alpha-specific genes in Saccharomyces yeast
A genomic code for nucleosome positioning
09/09/08
Yeast as a model organism
Ambhi and Raghav
Yeast as a model organism (Ppt presentation)
Papers:
1. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis
2. The original Yeast two hybrid paper
Data
To upload data, go [http://www.jhu.edu/iGEM/X_files/Read2.html here], click on [http://www.jhu.edu/iGEM/X_files/Read2.html upload data], and provide the necessary information and results.
How to submit data:
1. log-in as you once had to from the www.jhu.edu/iGEM website "login"
- User: ****** etc...
- Pass: ***** etc...
2. click on UPLOAD DATA from the 'x-files page'
3. add data etc.... and click submit: This generates a webpage and the URL to it is linked in the page you are directed to after you press submit. Copy that URL and past it into the wiki or into the web-browser url box to see what it looks like.
\* If you find that the picture you are uploading is not showing up e-mail Tejas.
Status Reports
The status reports of each group below will continuously be updated as we work on the biobricks. The following PDFs contain progressive versions of our status reports as we continue through the sex detector project; they are added weekly. To learn more about each biobrick, please refer to the Biobrick page.
Status Report 2.1 - 07/12/08
Status Report 2.2 - 07/17/08
Status Report 2.3 - 08/07/08
Please BOLD the most recent step that you have completed. Do this by placing the tag < b > in front of and </ b > at the end of what you would like to be placed in bold (with no space between letter and carrot symbol (<,>). Click on your group name for the detailed changes occurring to each biobrick.
GROUP 1: Fluorescent Proteins
Summary for Fluorescent Proteins Group
Date: Oct 19, 2008 Status report by: Tejas Part no.: BBa_K110017 -> BBa_K110023 Part Description: yESapphire , mCherry, venusYFP, and Citrine
Work on YFP has progressed, though the biobrick system is giving diffiulty in obtaining a useful construct. Moreover, the YFP sequence may not fully match the expected sequence. However, fluroescent proteins (yeast optimized) sent us as STABS from MIT are showing promise, and may be ready for some use by the Jamboree date if all goes well. Work will continue on venusYFP and mCherry (though mCherry unfortunately contains a restriction site). Sapphire must be started over sue to technical difficulties getting a clone.
GROUP 2: MATa Specific-promoters
Summary for MATa Specific Promoters Group
Date: August 19, 2008 Status report by: Allison and Nate Part no.: BBa_K110008 -> BBa_K110016 Part Description: A promoters: MFA1 (L+R) and Ste2 (R+L), respectively. Sequences were analyzed. BBa_K110016 had a perfect clone. Since there is not much miniprep left, a transformation was done to generate more clones with the correct sequence. BBa_K110008 had one mutation. Another transformation into a Kan BioBrick vector was attempted but was unsuccessful.
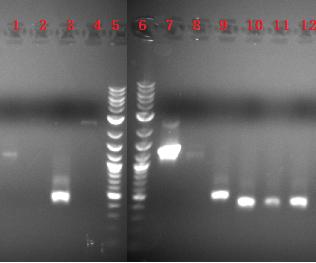
September 2: Transformation into a Cam BioBrick was completed, and the restriction digestion of the Biobricks from the pGEM vector were checked, comparing the inserts intended to be ligated to the Kan Biobrick vector and the inserts intended to be ligated to the Cam Biobrick vector; notice that the old inserts are either too faint to see or are not there: lanes2thru5OldDigest0816NewDigest0816 Lanes: 1 (2-log ladder), 2 BBa_K110008 old, 3 BBa_K110016 old, 4 BBa_K110008 new, 5 BBa_K110016 new, 6 (2-log ladder) A restriction digestion with EcoRI and PstI was done to check for the insert after ligated and transformed with the Cam Biobrick vector: 090308digest08_16_cam Lanes: 1 (old aliquot of 2-log ladder), 2 (new aliquot of 2-log ladder), 3 skip, 4 BBa_K110008, 5 skip, 6 BBa_K110016
September 7: Since the restriction digestions have been unclear as to whether or not the ligation of MFA1 and STE2 into the Cam BioBrick vector was successful, another transformation was done into the Cam Biobrick vector, restriction digestions on the previous mini prep from the 1st transformation and 2nd transformation completed, as well as PCR on the mini preps using the corresponding primers for BBa_K11008 and BBa_K110016. In the gel: after the 2 log ladder, the next for lanes have the restriction digestion products (approx 500 ng of plasmid each) and the second four lanes are pcr products in the following order: 08 (1st cam transformation) 16 (1st cam transformation) 08 (2nd transformation) 16 (2nd transformation) for both the restriction digestion and the pcr. The final lane was a control used in the pcr, unrelated to the project. 090708digestpcr0816 The restriction digestion did not show the insert, while pcr showed a band of the expected size in two of the four samples.
GROUP 3: Short Two-Way Stops
Summary for Short Two-Way Stops Group
Date:October 7,2008 Status report by: James Part no.:BBa_K110012;11;10;13;17 Direct transformation into Biobrick vectors has shown interesting results via analysis with PCR and cutting with Nsp1. The data is somewhat hard to decipher, but I am working on it...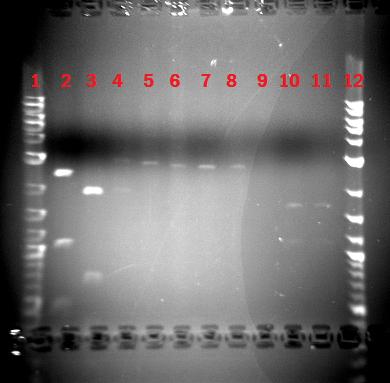
GROUP 4: Long Two-Way Stops & MAT(alpha) Specific Promotors
Summary for Long Two-Way Stops & MAT(alpha) Specific Promoters Group
Date: July 29, 2008 Status report by: Jaime Part no.: BBa_K110001, BBa_K110003, BBa_K110005, BBa_K110006 Part Description: Long Two-way Stops & Mat(alpha) specific promotors [http://www.jhu.edu/iGEM/Group4:LongTwo-wayStopsANDMATalphaSpecificPromoters/2008-7-25.Restriction%20Enzyme%20Digest%20of%20Mini-Preps.Jaime.html Restriction Enzyme Digest of Mini-Preps] Summary here: Need to be sequenced.
GROUP 5: MATa Specific Promoters II
Summary for MATa Specific Promoters II Group
Date: August 12, 2008 Status report by: Richard Group Part no.: BBa_K110009 Part Description: Ste2 Summary here: We hit a dead end when we ran out of the miniprep DNA. We are going to have to start again from scratch I suppose. Part no.: BBa_K110015 Part Description: Mfa1 Summary here: Same place as on Ste2.
GROUP 6: Vectors
Summary for Vectors Group
Date: July 31, 2008 Status report by: Tejas Part no.: BBa_K1100XX -> BBa_K1100YY Part Description: Vectors for concatenating and executing BioBricks The vectors to be used were delivered to us in STABS from MIT. They are pSB4A5 (amp), pSB4C5 (cam), pSB3K5 (kan), and pSB4K5 (kan). The vectors come pre-inserted with the ccdB gene, preventing growth in all E. coli strains except DB3.1. Total amount of DNA extracted from mini-prep is estimated between 5 to 20 ug per vector. Restriction digests confirmed the extracted DNA was vectors.([http://www.jhu.edu/iGEM/Group6:Vectors/2008-7-24.Vectors%20(CAM,AMP,KAN3,KAN4)%20after%20Digest.Tejas.html Digestion of all four vectors]). Vectors were transformed into JM109, BB#2198 (DB3.1), and BB#5777 (DB3.1). Alll gave expected results. 1 ug of each vector was digested for future use. Gel of digestion shows DNA is correct.([http://www.jhu.edu/iGEM/Group6:Vectors/2008-8-12.Digest%20of%20vectors,%20ready%20for%20distribution.Tejas.html Vectors ready for insertions])
GROUP 7: Microscopy/Yeast
Summary for Microscopy/Yeast Group
Date: _________ __, 2008 Status report by: _____ Part no.: BBa_K1100XX -> BBa_K1100YY Part Description: Summary here.
GROUP 8: Assembly Progress
Summary of Biobrick Assemblies:
Date:
 "
"