Team:Rice University/STRATEGY
From 2008.igem.org
(Difference between revisions)
DavidOuyang (Talk | contribs) |
StevensonT (Talk | contribs) (→Metabolic Parts) |
||
| Line 19: | Line 19: | ||
=== Metabolic Parts === | === Metabolic Parts === | ||
[[Image:TAL.png|right|190px|thumb|[http://www.rcsb.org/pdb/explore.do?structureId=1T6P TAL] monomer.]] | [[Image:TAL.png|right|190px|thumb|[http://www.rcsb.org/pdb/explore.do?structureId=1T6P TAL] monomer.]] | ||
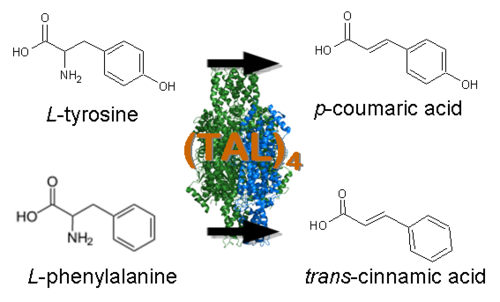
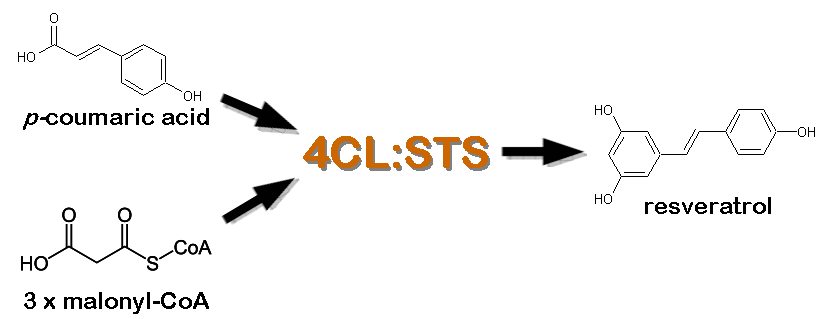
| - | *[http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&val=169745 Tyrosine Ammonia Lyase] (aka TAL, [http://partsregistry.org/wiki/index.php?title=Part:BBa_K122010 BBa_K122010]) - Begins the resveratrol biosynthetic pathway by catalyzing the the conversion of L-tyrosine to | + | *[http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&val=169745 Tyrosine Ammonia Lyase] (aka TAL, [http://partsregistry.org/wiki/index.php?title=Part:BBa_K122010 BBa_K122010]) - Begins the resveratrol biosynthetic pathway by catalyzing the the conversion of L-tyrosine to ''p''-coumaric acid and ammonia. TAL also exhibits Phenylalanine Ammonia Lyase (PAL) activity, converting L-phenylalanine to ''trans''-cinnamic acid and ammonia. Our work has focused on using [http://www.atcc.org/ATCCAdvancedCatalogSearch/ProductDetails/tabid/452/Default.aspx?ATCCNum=36575&Template=fungiYeast ''Rhodotorula glutinis''] TAL because its ratio of TAL to PAL activity is high compared with other TAL homologs. In addition, previous studies have shown that this enzyme can be expressed as a functional protein in ''Saccharomyces cerevisiae'' and ''Escherichia coli''. While the ''p''-coumaric acid produced by TAL will serve as a substrate for resveratrol biosynthesis, the ''trans''-cinnamic acid is expected to add a "floral" and "honey-like" bouquet to the beer. |
[[Image:TAL catalysis.png|left|500px]] | [[Image:TAL catalysis.png|left|500px]] | ||
Revision as of 22:09, 29 October 2008
| OUR TEAM ::: SUMMARY ::: INTRODUCTION ::: STRATEGY ::: RESULTS ::: ONGOING WORK ::: GALLERY
SUMMARY ::: BACKGROUND ::: STRATEGY ::: CONSTRUCTS ::: RESULTS ::: ONGOING WORK ::: OUR TEAM ::: NOTEBOOK ::: GALLERY |
 "
"