Team:UNIPV-Pavia/Protocols/Ligation
From 2008.igem.org
(Difference between revisions)
(New page: {| style="color:#000000;background-color:#ffffff;" cellpadding="3" cellspacing="1" border="3" bordercolor="#3128" width="80%" align="center" !align="center"|30px [[Team...) |
|||
| (8 intermediate revisions not shown) | |||
| Line 22: | Line 22: | ||
*[[Team:UNIPV-Pavia/Protocols/Digestion|BioBrick digestion with restriction enzymes]] | *[[Team:UNIPV-Pavia/Protocols/Digestion|BioBrick digestion with restriction enzymes]] | ||
*[[Team:UNIPV-Pavia/Protocols/GelExtraction|DNA gel extraction]] | *[[Team:UNIPV-Pavia/Protocols/GelExtraction|DNA gel extraction]] | ||
| + | *[[Team:UNIPV-Pavia/Protocols/Precipitation|DNA precipitation with sodium acetate]] | ||
*[[Team:UNIPV-Pavia/Protocols/AntarcticPhosphatase|Antarctic Phosphatase]] | *[[Team:UNIPV-Pavia/Protocols/AntarcticPhosphatase|Antarctic Phosphatase]] | ||
*[[Team:UNIPV-Pavia/Protocols/Ligation|Ligation]] | *[[Team:UNIPV-Pavia/Protocols/Ligation|Ligation]] | ||
| Line 28: | Line 29: | ||
<h1>Ligation</h1> | <h1>Ligation</h1> | ||
| - | ''(estimated time: | + | ''(estimated time: 20 min + 12-16 hours overnight incubation)'' |
<br> | <br> | ||
<br> | <br> | ||
'''Materials needed:''' | '''Materials needed:''' | ||
| - | *''' | + | *'''Roche T4 Ligase''' |
| - | *'''Buffer | + | *'''10X Roche T4 Ligase Buffer''' |
| - | + | ||
*'''ddH2O''' | *'''ddH2O''' | ||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| - | *For every | + | *(For every ligation) |
| - | ** | + | *Add 50 ng of vector |
| - | ** | + | *Add [[Image:pv_formula_lig.png]] |
| - | ** | + | *Heat DNA mix at 65°C for 5 min for DNA denaturation |
| - | * | + | *Add 1 µl of T4 Ligase buffer |
| + | *Add 1 µl of T4 Ligase | ||
| + | *10 µl final volume | ||
| + | *Incubate at 16°C overnight | ||
| + | <br> | ||
| + | *Then, ligation can be conserved at 4°C or can be transformed | ||
| + | *Before transformation you have to inactivate T4 Ligase: | ||
| + | **Heat ligation at 65°C for 10 min. | ||
<br> | <br> | ||
Latest revision as of 23:06, 1 October 2008
The protocols we used
- LB medium preparation
- Plasmid resuspension from IGEM paper spots
- Transformation
- Plasmid extraction
- BioBrick digestion with restriction enzymes
- DNA gel extraction
- DNA precipitation with sodium acetate
- Antarctic Phosphatase
- Ligation
- PCR
Ligation
(estimated time: 20 min + 12-16 hours overnight incubation)
Materials needed:
- Roche T4 Ligase
- 10X Roche T4 Ligase Buffer
- ddH2O
- (For every ligation)
- Add 50 ng of vector
- Add
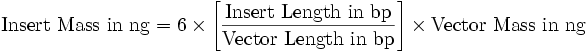
- Heat DNA mix at 65°C for 5 min for DNA denaturation
- Add 1 µl of T4 Ligase buffer
- Add 1 µl of T4 Ligase
- 10 µl final volume
- Incubate at 16°C overnight
- Then, ligation can be conserved at 4°C or can be transformed
- Before transformation you have to inactivate T4 Ligase:
- Heat ligation at 65°C for 10 min.
 "
"