Team:Hawaii/Initial BioBrick Plasmid Extraction From Filter Paper
From 2008.igem.org
Protocol
BioBricks parts used:
- BBa_E0040 GFP biobrick (spot 7E on plate 1001)
- BBa_J04430 IPTG-induced GFP device (spot 4H on plate 1004)
- BBa_I13522 constitutive GFP device (spot 8A on plate 1002)
Paper punch
- Warmed 5 μl aliquots of TE buffer (pH 8.0) to 50C in 0.5 ml PCR tubes
- Slid cutting mat under page in binder with desired part
- Pressed down w/ punch tool
- Ejected punch spot in TE buffer tube
Transformation
- Soaked spots 20 min in warmed TE buffer
- Thawed competent DH5α cells on ice. Chilled empty 2 ml eppendorf tubes on ice
- Added 2 μl TE + DNA solution to 50 μl thawed cells
- Incubated 30 min on ice
- Heat shocked in 42C water bath for 60 sec
- Incubated 2 min on ice
- Added 200 μl SOC
- Incubated for 2 hr at 37C with shaking (235 rpm)
- Plated 200 μl 1/1, 1/10, 1/100 dilutions on LB+amp
- Incubated overnight at 37C.
Protocol adapted from Registry of Standard Biological Parts, [http://partsregistry.org/wiki/index.php/Help:IGEM_08_DNA_distribution Spring 2008 DNA Distribution].
Results
One colony was observed on the 1/1 dilution plate for GFP. Three colonies were observed on the 1/1 dilution plate for lacI/IPTG induced GFP.
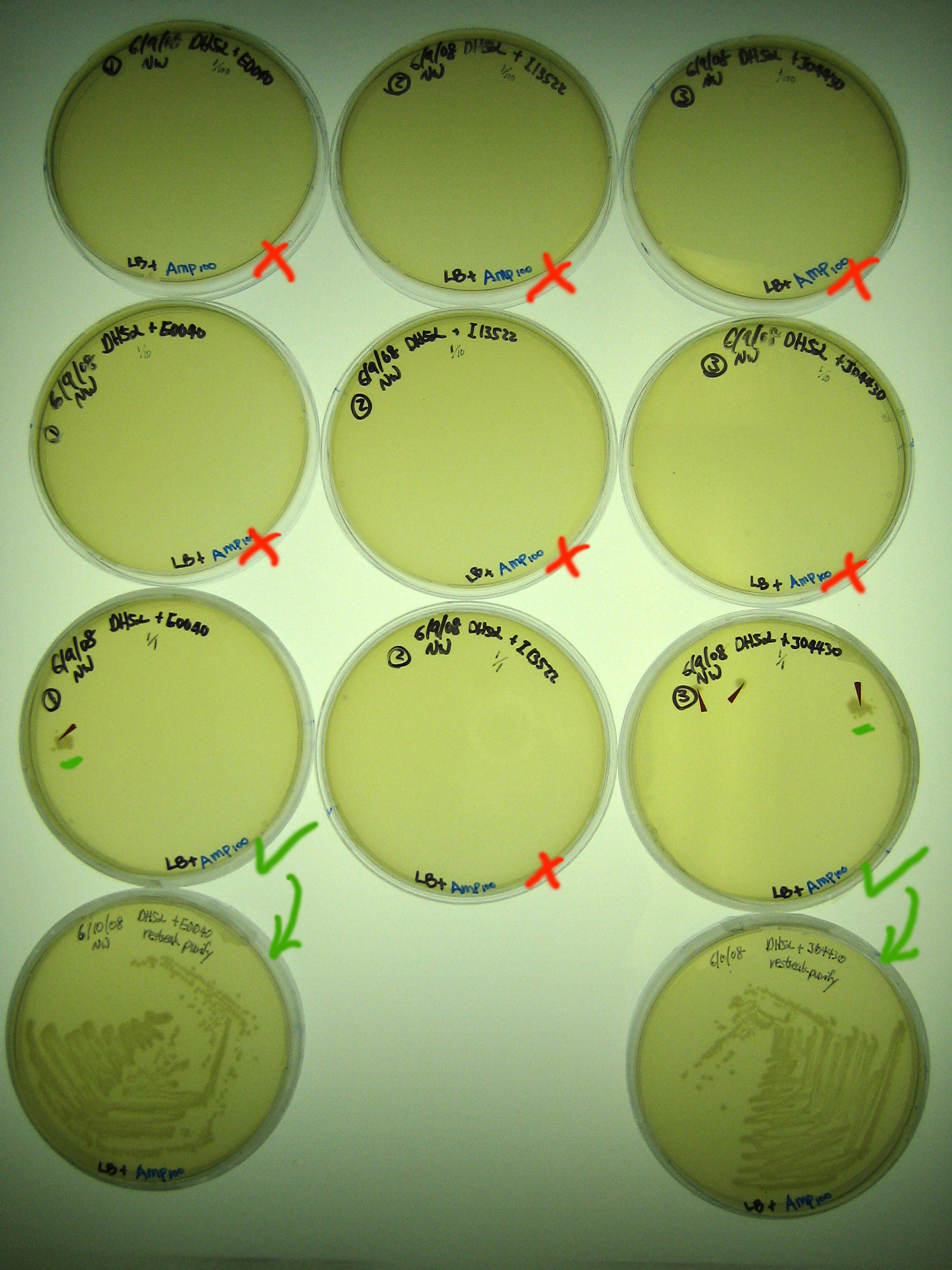
Discussion
Need to incubate ~20 hours for small/medium colonies to appear. Colonies that did arise are unlikely to be random mutants that carry ampR because amp is a bactericidal antibiotic.
Is the low number of transformants due to low competency of our cells or low concentration of DNA extracted from iGEM Biobricks filter paper? Norman will find out concentration of Biobricks on filter paper.
GP suggested that we mush up the filter paper to extract as much DNA as possible. Also, we should add 7 μl 1X TE buffer (instead of the recommended 5 μl) if we intend on doing two transformations that day. The filter paper absorbs a lot of the solution. It's probably not possible to freeze/thaw this back if we leave the filter paper in because all the TE+DNA gets reabsorbed (unless we add more TE).
We will do another Biobrick extraction/transformation for competency comparison between our cells (not so competent) and commercial super competent cells. (see experiment)
 "
"