DNA-Origami
From 2008.igem.org
(Difference between revisions)
m |
|||
| (One intermediate revision not shown) | |||
| Line 55: | Line 55: | ||
<h3><span style="font-weight: bold;">Origami</span></h3> | <h3><span style="font-weight: bold;">Origami</span></h3> | ||
| - | <h4><span style="font-weight: bold;"></span>Origami<br></h4> | + | <h4><span style="font-weight: bold;"></span>Origami production<br></h4> |
To produce the Origami we mixed each the M13mp18 DNA with the oligos, | To produce the Origami we mixed each the M13mp18 DNA with the oligos, | ||
| Line 72: | Line 72: | ||
the 2 oligos with the Alexa 488 were used.<br> | the 2 oligos with the Alexa 488 were used.<br> | ||
<br> | <br> | ||
| - | <h4><span style="font-weight: bold;"></span>Purification of | + | <h4><span style="font-weight: bold;"></span>Purification of DNA-Origamis<br> |
</h4> | </h4> | ||
To purify the DNA-Origamis from the unbound DNA-oligos, we used Montage® PCR Centrifugal Filter Devices (Millipore). The Montage® PCR Centrifugal Filter Devices were labeled and put with the purple side on top in 1.5 ml Eppendorf tubes. To clean the filter of remaining glycerol, 450 µl TAE/MgAcetat (12.5 mM; 1x filtered) was put on top of the filter and centrifuged for 15 min at 1000 g. After removing the filtrate, 400 µl TEA/MgAcetat (12.5 mM;1x filtered) and 45 µl DNA-origami were put on top of the filter and again centrifuged for 15 min at 1000 g. The filtrate was removed again. All unbound DNA-oligos were washed off by putting 400 µl TEA/MgAcetat (12.5 mM; 1x filtered) on top of the filter. The sample was centrifuged for 15 min at 1000 g. To release the DNA-origamis of the filter, 100 µl TAE/MgAcetat (12.5 mM;1x filtered) was put on top of the filter, and the filter was left at room temperature for at least 2 min. The filter shouldn´t run dry. The Montage® PCR Centrifugal Filter Devices were put upside down (the purple side has to be on the bottom) in one of the special Invert Spin tubes form Millipore and centrifuged for 3 min at 1000 g. | To purify the DNA-Origamis from the unbound DNA-oligos, we used Montage® PCR Centrifugal Filter Devices (Millipore). The Montage® PCR Centrifugal Filter Devices were labeled and put with the purple side on top in 1.5 ml Eppendorf tubes. To clean the filter of remaining glycerol, 450 µl TAE/MgAcetat (12.5 mM; 1x filtered) was put on top of the filter and centrifuged for 15 min at 1000 g. After removing the filtrate, 400 µl TEA/MgAcetat (12.5 mM;1x filtered) and 45 µl DNA-origami were put on top of the filter and again centrifuged for 15 min at 1000 g. The filtrate was removed again. All unbound DNA-oligos were washed off by putting 400 µl TEA/MgAcetat (12.5 mM; 1x filtered) on top of the filter. The sample was centrifuged for 15 min at 1000 g. To release the DNA-origamis of the filter, 100 µl TAE/MgAcetat (12.5 mM;1x filtered) was put on top of the filter, and the filter was left at room temperature for at least 2 min. The filter shouldn´t run dry. The Montage® PCR Centrifugal Filter Devices were put upside down (the purple side has to be on the bottom) in one of the special Invert Spin tubes form Millipore and centrifuged for 3 min at 1000 g. | ||
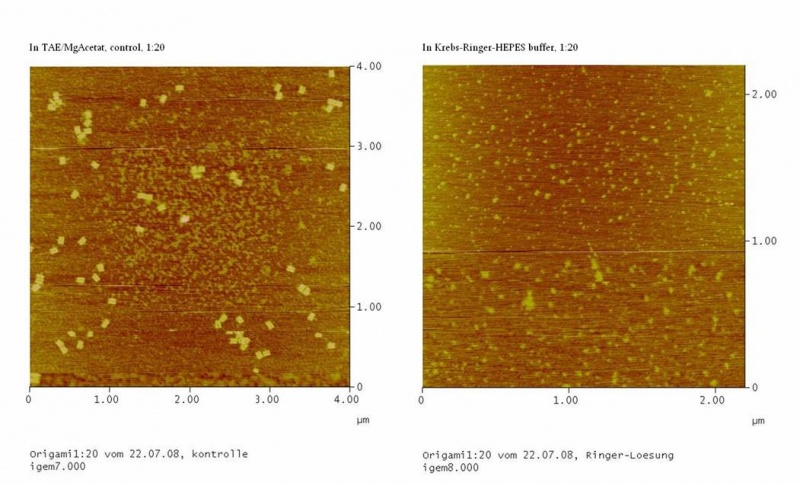
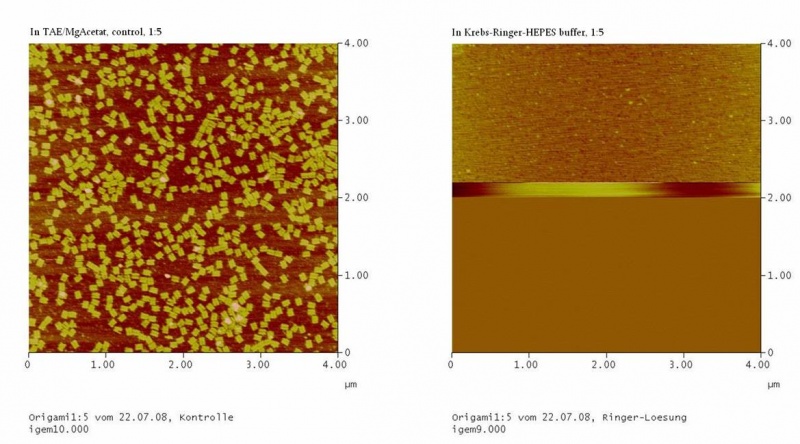
The Origami were kept in different buffers. For this, TEA/MgAcetat (12.5 mM; 1x filtered) was replaced by the according buffer.<br> | The Origami were kept in different buffers. For this, TEA/MgAcetat (12.5 mM; 1x filtered) was replaced by the according buffer.<br> | ||
| - | <h4><span style="font-weight: bold;"></span>Atomic force microscopy to | + | <h4><span style="font-weight: bold;"></span>Atomic force microscopy to test the origami stability |
<br> | <br> | ||
</h4> | </h4> | ||
| Line 83: | Line 83: | ||
The metal pane was fixed in the metal sample holder by a magnet, so that the sample could not move itself during the measurement. | The metal pane was fixed in the metal sample holder by a magnet, so that the sample could not move itself during the measurement. | ||
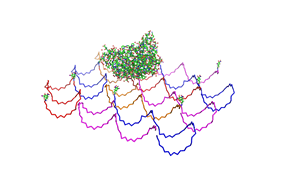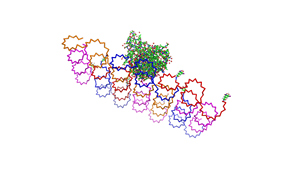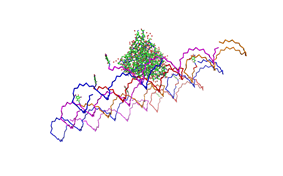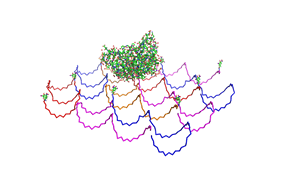
<h2>Results and Discussion</h2> | <h2>Results and Discussion</h2> | ||
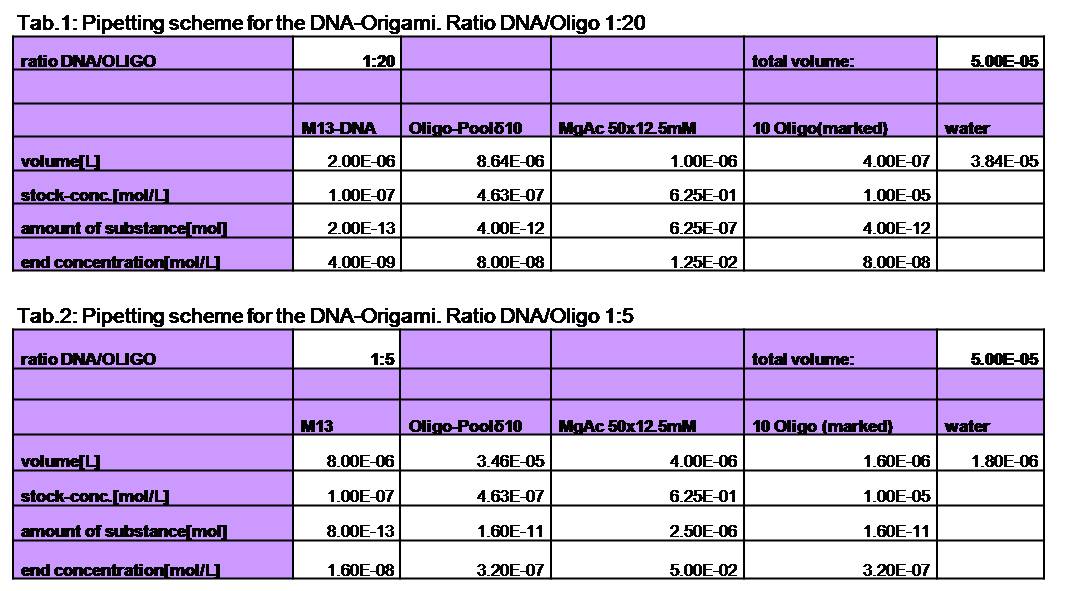
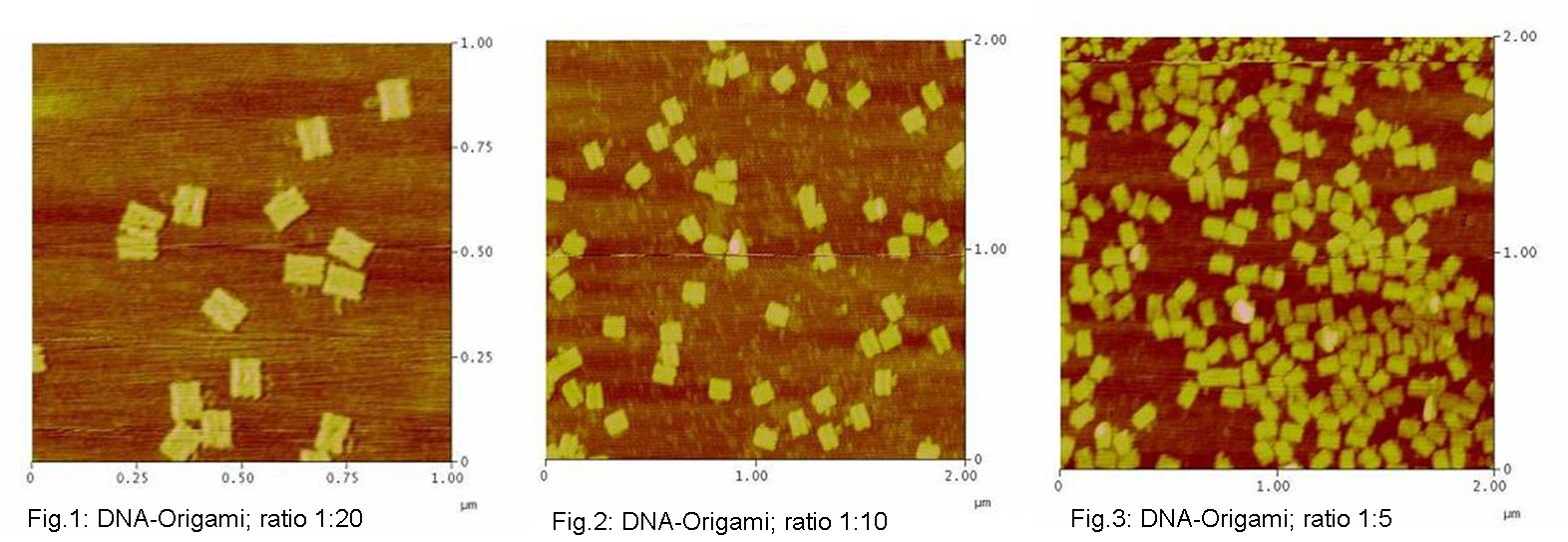
| - | <h3>1. Different ratios of | + | <h3>1. Different ratios of phage DNA to staple oligonucleotides</h3> |
Because we also wanted to measure the calcium influx in the LSRII | Because we also wanted to measure the calcium influx in the LSRII | ||
fluorescence spectrometer, we had to increase the concentration of the | fluorescence spectrometer, we had to increase the concentration of the | ||
Origamis at least up to 200 nM. Because the oligos we had to use for | Origamis at least up to 200 nM. Because the oligos we had to use for | ||
| - | building | + | building DNA-Origami are very expensive, we first tried to reduce the |
| - | ratio of | + | ratio of phage DNA to oligos. Therefore we tried to make Origamis with two |
(1:10) and four (1:5) times lower concentration of oligos. As positive | (1:10) and four (1:5) times lower concentration of oligos. As positive | ||
control we took the 1:20 ratio at which we knew it should be stable. We | control we took the 1:20 ratio at which we knew it should be stable. We | ||
| - | used | + | used AFM to check if the Origami are well formed. The results are |
shown in figures 1-3. <br> | shown in figures 1-3. <br> | ||
<br> | <br> | ||
Latest revision as of 01:50, 30 October 2008
 "
"