Team:Edinburgh/Plan/Beta-Carotene
From 2008.igem.org
(→Directly involved genes from P. ananatis) |
(→Assembley) |
||
| (5 intermediate revisions not shown) | |||
| Line 17: | Line 17: | ||
== Indirectly involved genes from ''E. coli'' == | == Indirectly involved genes from ''E. coli'' == | ||
| + | |||
| + | :* 1-deoxyxylulose-5-phosphate synthase ([https://2008.igem.org/Team:Edinburgh/dxs '''''dxs''''']) catalyses synthesis of 1-deoxy-D-xylulose-5-phosphate from glyceraldehyde-3-phosphate and pyruvate, transferring these two substrates from the glycolysis pathway to the lycopene synthesis pathway (see overview). It has been identified as a rate limiting step. To overcome this we decided to make a ''dxs'' BioBrick<sup>TM</sup> in order to increase the gene copy number. | ||
| + | :* ([https://2008.igem.org/Team:Edinburgh/appY '''''appY''''']) encodes a transcriptional regulator related to anaerobic energy metabolism. It is not directly involved in the lycopene synthesis pathway, but co-expression of ''appY'' with ''dxs'' has been reported to produce 8x the amount of lycopene produced in the absence of these genes. | ||
| + | |||
| + | == Assembly == | ||
| + | |||
| + | In order to maximise production, we decided that the genes should be as an operon in the order ''dxs'', ''crtE'', ''crtB'', ''crtI'', ''crtY'', ''appY''. This, with the exception of ''appY'', reflects the order in which the enzymes act, and thus their importance. We consider ''appY'' to be the least essential of the genes, and thus it is suitable for being at the end of the operon, by which time it is possible that transcription in many cases will have ceased. | ||
| + | |||
| + | As with the starch synthesis genes, these genes should be under the control of a constitutively active promoter. However, if testing of them shows that more than enough β-carotene is being produced, it would be advisable to replace the constitutive promoter with a weaker one, allowing more energy and glucose to be channeled down the starch synthesis pathway. | ||
== Overview == | == Overview == | ||
Latest revision as of 02:01, 30 October 2008
Contents |
β-carotene synthesis
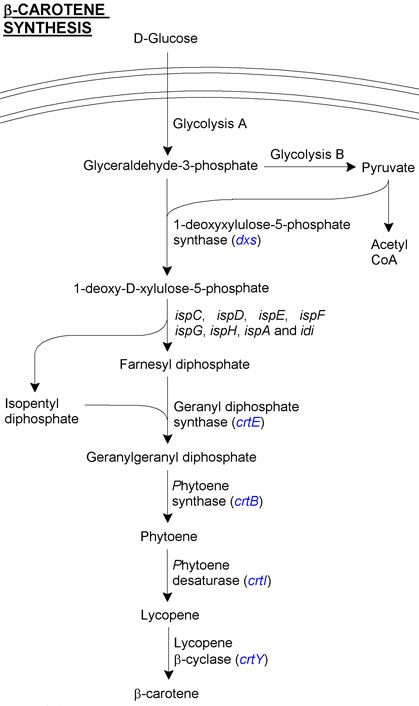
β-carotene is produced from the products of glycolysis, as can be seen in the overview figure. We have concentrated on two areas of β-carotene for our project. The first involves transfering carotenoid synthesis genes from Pantoea ananatis, a member of the proteobacteria naturally capable of producing β-carotene. The second involves upregulating the glycolysis pathways in E. coli in order to concentrate more energy into making β-carotene.
Directly involved genes from P. ananatis
- Geranyl diphosphate synthase (crtE) converts the substrates farnesyl diphosphate and isopentyl diphosphate into geranyl geranyl diphosphate.
- Geranyl geranyl diphosphate is then converted into phytoene by phytoene synthase (crtB).
- Lycopene is produced from phytoene by phytoene desaturase (crtI).
- Finally, lycopene β-cyclase (crtY) cyclises lycopene to produce β-carotene.
See figure 1 for structure details.
Indirectly involved genes from E. coli
- 1-deoxyxylulose-5-phosphate synthase (dxs) catalyses synthesis of 1-deoxy-D-xylulose-5-phosphate from glyceraldehyde-3-phosphate and pyruvate, transferring these two substrates from the glycolysis pathway to the lycopene synthesis pathway (see overview). It has been identified as a rate limiting step. To overcome this we decided to make a dxs BioBrickTM in order to increase the gene copy number.
- (appY) encodes a transcriptional regulator related to anaerobic energy metabolism. It is not directly involved in the lycopene synthesis pathway, but co-expression of appY with dxs has been reported to produce 8x the amount of lycopene produced in the absence of these genes.
Assembly
In order to maximise production, we decided that the genes should be as an operon in the order dxs, crtE, crtB, crtI, crtY, appY. This, with the exception of appY, reflects the order in which the enzymes act, and thus their importance. We consider appY to be the least essential of the genes, and thus it is suitable for being at the end of the operon, by which time it is possible that transcription in many cases will have ceased.
As with the starch synthesis genes, these genes should be under the control of a constitutively active promoter. However, if testing of them shows that more than enough β-carotene is being produced, it would be advisable to replace the constitutive promoter with a weaker one, allowing more energy and glucose to be channeled down the starch synthesis pathway.
Overview
- In blue are the genes which we manipulated.
 "
"