Team:Illinois/Antibody GPCR Fusion Notebook
From 2008.igem.org
(Difference between revisions)
(→9th October) |
(→Primers) |
||
| (31 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
** 200uL 0.5M EDTA (1mM) | ** 200uL 0.5M EDTA (1mM) | ||
| + | |||
| + | ==Primers== | ||
| + | |||
| + | PGK promoter | ||
| + | Fwd:5'TTTT GAATTC AAAGATGCCGATTTGGGCGC | ||
| + | Rev:5'TTTT GAGCTC GTTTTATATTTGTTGTAAAA | ||
| + | |||
| + | PGK terminator | ||
| + | Fwd:5'TTAT GGGCCC GAAATAAATTGAATTGAATT | ||
| + | Rev:5'TTTTG AAGCTT CAGCTTTAACGAACGCAGA | ||
| + | |||
| + | Ste2 | ||
| + | Fwd:5'GCCC TCTAGA ATGTCTGATGCGGCTCCTTC | ||
| + | Rev:5'TTAT GGGCCC TCATAAATTATTATTATCTT | ||
| + | |||
| + | Fus1 upstream | ||
| + | Fwd:5'GTGG GAATTC TAATAATCAGAACTCCAACA | ||
| + | Rev:5'GGCG TCTAGA TTTGATTTTCAGAAACTTGA | ||
| + | |||
| + | Fus1 downstream: | ||
| + | Fwd:5'GCGA GGTACC TGAAAATAATATTGACGTTC | ||
| + | Rev:5'TTAT GCGGCCGC TATTCACCAGACCCGCTCCT | ||
== 22nd July == | == 22nd July == | ||
| Line 105: | Line 127: | ||
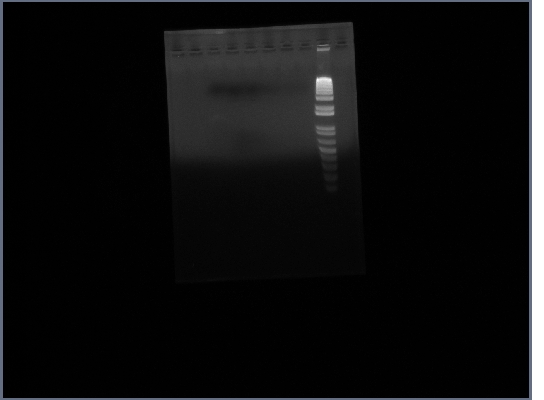
* Ran gel of PCR products (1.5% agarose, 200V) | * Ran gel of PCR products (1.5% agarose, 200V) | ||
** Result: No bands present | ** Result: No bands present | ||
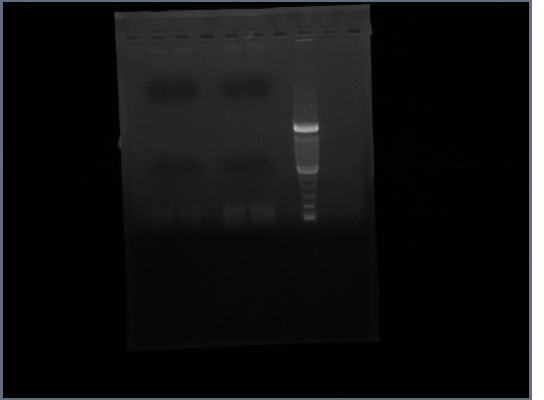
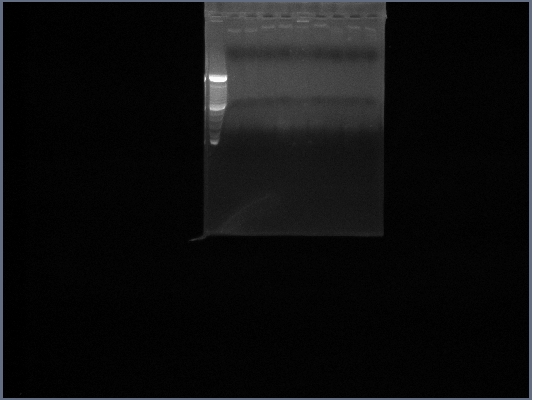
| + | [[Image:GPCR8-28.jpg|190px]] | ||
== 2nd September == | == 2nd September == | ||
| Line 148: | Line 171: | ||
*** Too low | *** Too low | ||
** 50 minutes | ** 50 minutes | ||
| - | *Poor results | + | *Poor results--no bands present |
== 8th September == | == 8th September == | ||
| Line 182: | Line 205: | ||
* Signs of life in 3 of the cultures | * Signs of life in 3 of the cultures | ||
** Wait until tomorrow | ** Wait until tomorrow | ||
| - | * Ran gel on PCR from 8th September | + | * Ran gel on PCR from 8th September--no bands present |
** 150V, 50 minutes | ** 150V, 50 minutes | ||
** No sign of DNA | ** No sign of DNA | ||
* Ladder from Courtney | * Ladder from Courtney | ||
| - | |||
== 10th September == | == 10th September == | ||
* Split culture | * Split culture | ||
| - | * Ran gel from 8th September again | + | * Ran gel from 8th September again--no bands present |
** 150V, 50 minutes | ** 150V, 50 minutes | ||
** 0.75% gel | ** 0.75% gel | ||
| Line 455: | Line 477: | ||
**same protocol as 23rd September | **same protocol as 23rd September | ||
**Templates 2a, 3a, 4a used (3 reactions each) | **Templates 2a, 3a, 4a used (3 reactions each) | ||
| + | |||
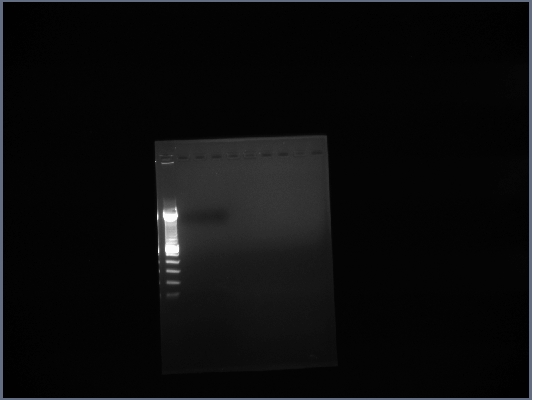
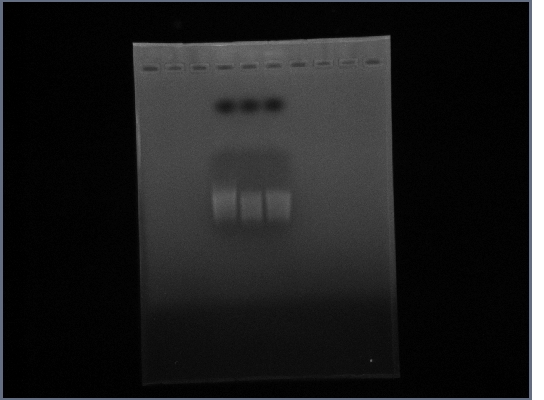
| + | ==3rd October== | ||
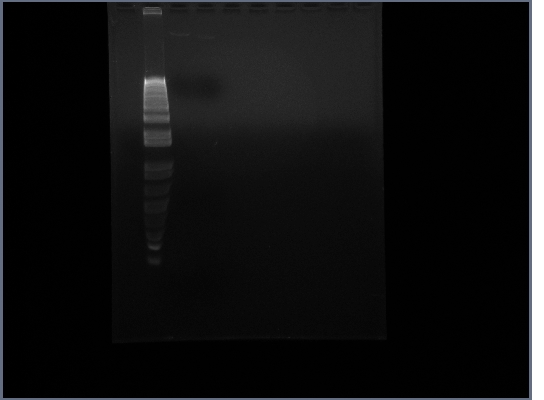
| + | * Ran Ste2 gel from 1st October | ||
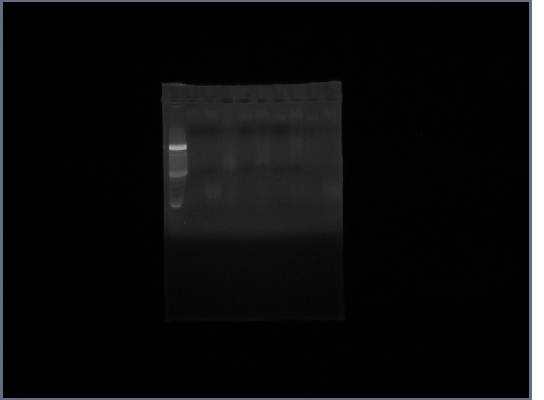
| + | [[Image:GPCR10-3.jpg|190px]] | ||
== 8th October == | == 8th October == | ||
| Line 503: | Line 529: | ||
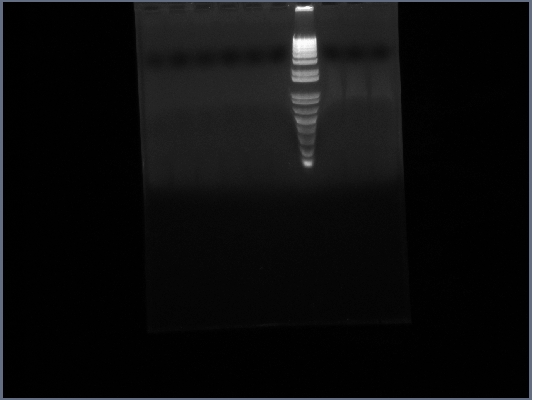
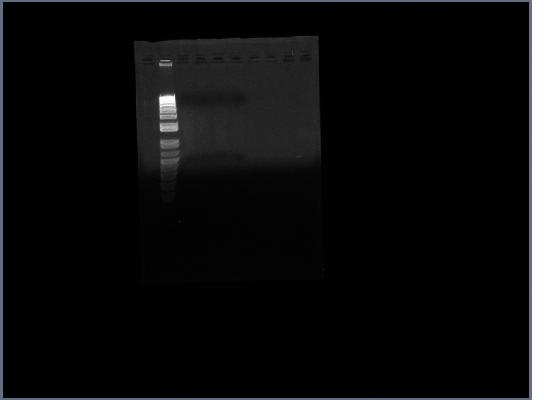
* Ran Ste2 gel from today | * Ran Ste2 gel from today | ||
[[Image:GPCR10-09Ste2.jpg|190px]] | [[Image:GPCR10-09Ste2.jpg|190px]] | ||
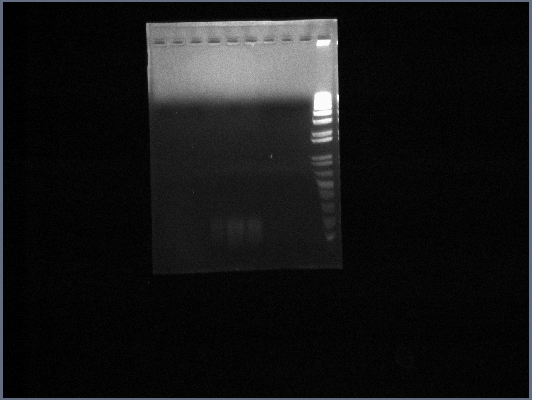
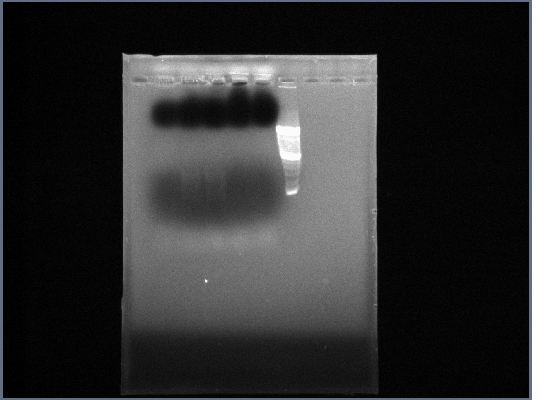
| + | * Ran PGK terminator gel from yesterday | ||
| + | [[Image:GPCR10-09PGKp.jpg|190px]] | ||
== 12th October == | == 12th October == | ||
* PCR: Fus1 (3 reactions each) | * PCR: Fus1 (3 reactions each) | ||
| + | **Template is products from 9th October | ||
== 13th October == | == 13th October == | ||
| - | * Ran PCR with Fus1 | + | * Ran gel of PCR with Fus1 |
* Used 25uL or product, 5uL of loading dye | * Used 25uL or product, 5uL of loading dye | ||
| + | [[Image:GPCR10-13.jpg|190px]] | ||
== 14th October == | == 14th October == | ||
| Line 540: | Line 570: | ||
|} | |} | ||
* Template is reaction from 12th October | * Template is reaction from 12th October | ||
| - | |||
| - | |||
| - | |||
| - | |||
== 15th October == | == 15th October == | ||
| - | |||
*PCR PGK Terminator | *PCR PGK Terminator | ||
{| class="wikitable" border="1" | {| class="wikitable" border="1" | ||
| Line 566: | Line 591: | ||
|} | |} | ||
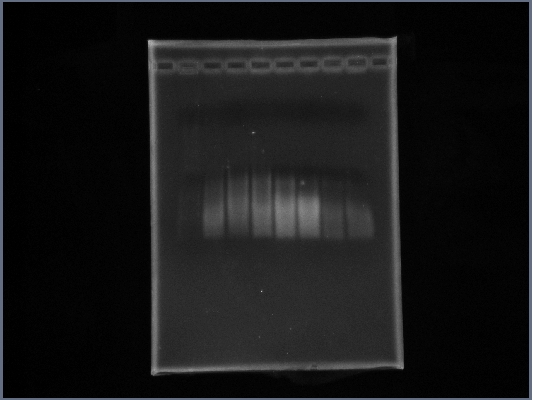
| - | * Ran gel of Ste2 from | + | *Ran gel of Ste2 from 10/14 |
| - | * | + | [[Image:GPCR10-15Ste2.jpg|190px]] |
| + | *Ran gel of Fus1 upstream from 10/14 (no ladder, oops) | ||
| + | [[Image:GPCR10-15.jpg|190px]] | ||
== 16th October == | == 16th October == | ||
| Line 595: | Line 622: | ||
** Tube 4: Template 4a - PGK Promoter | ** Tube 4: Template 4a - PGK Promoter | ||
| - | * Extracted Ste2 | + | *ran Gel of above |
| + | [[Image:GPCR10-16Fusd.jpg|190px]] | ||
| + | |||
| + | *ran Gel of PGK terminator from 10/15 | ||
| + | [[Image:GPCR10-16.jpg|190px]] | ||
| + | |||
| + | |||
| + | |||
| + | * Extracted Ste2 from 10/15 and re-amplified: | ||
{| class="wikitable" border="1" | {| class="wikitable" border="1" | ||
| Line 614: | Line 649: | ||
|9uL | |9uL | ||
|} | |} | ||
| - | |||
| - | |||
== 17th October == | == 17th October == | ||
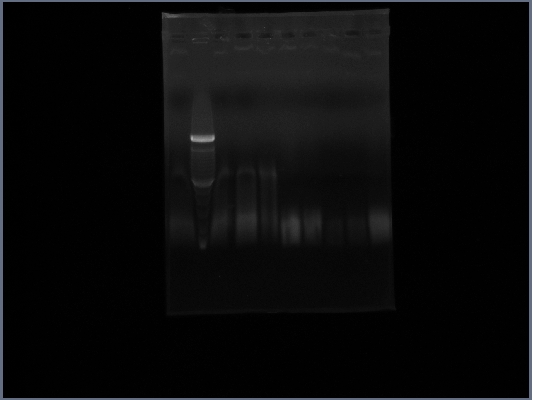
| - | * Ran gel | + | * Ran gel of Ste2 from 10/16 |
** Tube 1 - lane 2,3 | ** Tube 1 - lane 2,3 | ||
** Tube 2 - lane 5,6 | ** Tube 2 - lane 5,6 | ||
| + | [[Image:GPCR10-17.jpg|190px]] | ||
| - | * PCR: Fus1 | + | * PCR: Fus1 upstream |
{| class="wikitable" border="1" | {| class="wikitable" border="1" | ||
|- | |- | ||
| Line 640: | Line 674: | ||
|9uL | |9uL | ||
|} | |} | ||
| + | **template is products from 10/14 | ||
* PCR: PGK Terminator | * PCR: PGK Terminator | ||
| - | |||
{| class="wikitable" border="1" | {| class="wikitable" border="1" | ||
|- | |- | ||
| Line 660: | Line 694: | ||
|9uL | |9uL | ||
|} | |} | ||
| + | **Template is products from 10/15 | ||
* Extracted Ste2(Gel from today) | * Extracted Ste2(Gel from today) | ||
| + | |||
| + | == 18th October== | ||
| + | Ran gel of Fus1 upstream and PGK terminator from yesterday | ||
| + | [[Image:GPCR10-18.jpg|190px]] | ||
== 20th October == | == 20th October == | ||
| Line 700: | Line 739: | ||
* Ran gel on Ste2 from today | * Ran gel on Ste2 from today | ||
| + | [[Image:GPCR10-21.jpg|190px]] | ||
== 22nd October == | == 22nd October == | ||
| Line 762: | Line 802: | ||
* Ran gel of Fus1 Upstream, PGK Terminator, Ste2 from yesterday | * Ran gel of Fus1 Upstream, PGK Terminator, Ste2 from yesterday | ||
** 1% Agarose | ** 1% Agarose | ||
| + | [[Image:GPCR10-23.jpg|190px]] | ||
* PCR: Fus1 Downstream, PGK Promoter | * PCR: Fus1 Downstream, PGK Promoter | ||
| Line 783: | Line 824: | ||
** 4 reactions each of Fus1 Upstream(1,2,3,4) and PGK Promoter(A,B,C,D) | ** 4 reactions each of Fus1 Upstream(1,2,3,4) and PGK Promoter(A,B,C,D) | ||
| + | *Gel shows no bands. | ||
| + | |||
* PCR: Fus1 Downstream, Fus1 Upstream, PGK Promoter, PGK Terminator, Ste2 | * PCR: Fus1 Downstream, Fus1 Upstream, PGK Promoter, PGK Terminator, Ste2 | ||
| - | ** Template from 20th October | + | ** Template genomic DNA from 20th October (x4 different reactions) |
** Used all Template | ** Used all Template | ||
| Line 805: | Line 848: | ||
|9uL | |9uL | ||
|} | |} | ||
| + | *Gel shows no bands | ||
| + | |||
| + | ==24th October== | ||
| + | *Ran gel of Fus1 upstream(lanes 3,4,5), PGK terminator(lanes6,7), and Ste2(lanes 8,9) from 10/22 | ||
| + | **ladder is lane 2; 1 and 10 are nothing | ||
| + | [[Image:GPCR10-22.jpg|190px]] | ||
| + | |||
| + | *PCR of Fus1 downstream, Fus1 upstream, PGK promoter, PGK terminator, Ste2 | ||
| + | {| class="wikitable" border="1" | ||
| + | |- | ||
| + | |MasterMix | ||
| + | |20uL | ||
| + | |- | ||
| + | |Primer Fwd | ||
| + | |0.5uL | ||
| + | |- | ||
| + | |Primer Rev | ||
| + | |0.5uL | ||
| + | |- | ||
| + | |Template | ||
| + | |29uL | ||
| + | |} | ||
| + | **The templates were the rest of the genomic DNA extracts from 9/12 | ||
| + | **Three reactions of each gene were run | ||
| + | |||
| + | *Gel from above: | ||
| + | [[Image:GPCR10-24.jpg|190px]][[Image:GPCR10-24(cont.).jpg|190px]] | ||
| + | *(on left)Fus1 downstream lanes 2,3,4; Fus1 upstream lanes 5,6,7; PGK promoter lanes 8,9,10; | ||
| + | *(on right)PGK terminator lanes2,3,10; Ste2 lanes 4,5,6; Fus1 downstream lanes 7,8,9; | ||
| + | |||
| + | ==25th October== | ||
| + | *Extracted DNA from the gel from 10/24 | ||
| + | **FUS1 upstream from the higher bands of lanes 3 and 4 | ||
| + | **PGK terminator from the lower bands of lanes 6 and 7 | ||
| + | *DNA ligation | ||
| + | {| class="wikitable" border="1" | ||
| + | |- | ||
| + | |DNA | ||
| + | |5uL | ||
| + | |- | ||
| + | |buffer | ||
| + | |5uL | ||
| + | |- | ||
| + | |Re1 (Pst1) | ||
| + | |1uL | ||
| + | |- | ||
| + | |Re2 (EcoR1) | ||
| + | |1uL | ||
| + | |- | ||
| + | |Water | ||
| + | |37.5uL | ||
| + | |} | ||
| + | |||
| + | ==26th October== | ||
| + | *Incubate for 20mins at 80 degrees celcius | ||
| + | {| class="wikitable" border="1" | ||
| + | |- | ||
| + | |Ligation Buffer | ||
| + | |4uL | ||
| + | |- | ||
| + | |DNA Ligase | ||
| + | |1uL | ||
| + | |- | ||
| + | |DNA | ||
| + | |3uL | ||
| + | |- | ||
| + | |Plasmid | ||
| + | |9uL | ||
| + | |- | ||
| + | |Water | ||
| + | |3uL | ||
| + | |} | ||
| + | *Let sit for 5 min. | ||
| + | *5uL of above mixture to competent cells | ||
| + | **30 min. on ice | ||
| + | *Heat shock 30s (42 degrees) | ||
| + | *Add SOC Media (200uL) | ||
| + | **Incubate 60 min.(37 degrees) | ||
| + | *Plate 200uL | ||
| + | **Incubate 37 degrees celcius overnight | ||
| + | |||
| + | ==27th October== | ||
| + | The transformation failed; try again with the same protocol: | ||
| + | *Using extracts 3 and 7 (+Ligation buffers, Ligase, Plasmid, and Water) | ||
| + | *Failed again. | ||
<!-- == Insert Date Here == | <!-- == Insert Date Here == | ||
Latest revision as of 02:55, 30 October 2008
| Home | Team | Project | Notebook | Research Articles | Parts | Protocols | Pictures |
Recipes
- Tris-Cl, 1M
- Dissolve 121g Tris base in 800ml H2O
- Adjust to desired pH with concentrated HCl
- Mix and add H2O to 1 liter
- (Approximately, 20ml HCl for pH 7.4 and 42ml for pH 8.0)
- EDTA, 0.5M (pH 8.0)
- Dissolve 186.1g Na2 EDTA-2H2O in 700ml H20
- Adjust pH to 8.0 with 10M NaOH(~50ml)
- Add H2O to 1 liter
- Breaking buffer - 100ml
- 2ml Triton X-100
- 1ml Sodium dodecyl sulfate (SDS)
- 0.5844g NaCl (100mM)
- 1ml 1M Tris-Cl pH 8.0 (10mM)
- 200uL 0.5M EDTA (1mM)
Primers
PGK promoter Fwd:5'TTTT GAATTC AAAGATGCCGATTTGGGCGC Rev:5'TTTT GAGCTC GTTTTATATTTGTTGTAAAA
PGK terminator Fwd:5'TTAT GGGCCC GAAATAAATTGAATTGAATT Rev:5'TTTTG AAGCTT CAGCTTTAACGAACGCAGA
Ste2 Fwd:5'GCCC TCTAGA ATGTCTGATGCGGCTCCTTC Rev:5'TTAT GGGCCC TCATAAATTATTATTATCTT
Fus1 upstream Fwd:5'GTGG GAATTC TAATAATCAGAACTCCAACA Rev:5'GGCG TCTAGA TTTGATTTTCAGAAACTTGA
Fus1 downstream: Fwd:5'GCGA GGTACC TGAAAATAATATTGACGTTC Rev:5'TTAT GCGGCCGC TATTCACCAGACCCGCTCCT
22nd July
- Yeast obtained from Dr. Zhao
- W303a S. Cerevisiae
24th July
- Prepared liquid culture for DNA extraction
- Made 1M Tris. Cl pH 8.0
- Made 4M ammonium acetate
22nd August
- Attempted DNA extraction of W303a genomic DNA
- Protocol from Wiley's Current Protocols in Molecular Biology
- Result: Failed to finish protocol
- Obtained more yeast from Dr. Zhao
- W303a S. cerevisiae
25th August
- Prepared overnight culture for DNA extraction (3:27pm)
26th August
- Attempted DNA extraction
- Prepped overnight culture
27th August
- Performed PCR: PGK Terminator
| Buffer G | 12.5uL | x4 | 50uL | |
| Forward Primer | 0.5uL | x4 | 2uL | |
| Reverse Primer | 0.5uL | x4 | 2uL | |
| H2O | 10.8uL | x4 | 43.2uL | |
| Taq | 0.2uL | x4 | 0.8uL | |
| template | 0.5ul | |||
| Negative control | 3 H2O | |||
- PCR program:
- 4 min 94 degrees
- 25-30x 30s 94 degrees
- 30s Tm primers
- 1 min/KB 72 degrees
- 7 min 72 degrees
- For the gel: 5uL loading dye gel is in cold room
- Prepped 3 overnight cultures
28th August
- Extracted DNA from 4 cultures
- Ran gel of PCR products (1.5% agarose, 200V)
- Result: No bands present
2nd September
- PCR: PGK Terminator
| 5 PRIME Mastermix | 10uL | x3 | 30uL |
| Forward Primer | 0.5uL | x3 | 1.5uL |
| Reverse Primer | 0.5uL | x3 | 1.5uL |
| Template | 10uL | x3 | 30uL |
| H2O | 10.8uL | x4 | 43.2uL |
| Negative control | 25uL H2O |
3rd September
- Ran gel
- Ladder lane 7
- Sample 7 spilled
- 1% agarose
- 120V
- Too low
- 50 minutes
- Poor results--no bands present
8th September
- PCR: PGK Terminator
| 5PRIME Mastermix | 10uL | x3 | 30uL |
| Forward Primer | 0.5uL | x3 | 1.5uL |
| Reverse Primer | 0.5uL | x3 | 1.5uL |
| Template | 14uL | x3 | 42uL |
- Prepped 4 overnight cultures
- Yeast dried out again
9th September
- Signs of life in 3 of the cultures
- Wait until tomorrow
- Ran gel on PCR from 8th September--no bands present
- 150V, 50 minutes
- No sign of DNA
- Ladder from Courtney
10th September
- Split culture
- Ran gel from 8th September again--no bands present
- 150V, 50 minutes
- 0.75% gel
- Ladder from Courtney
11th September
- PCR: PGK Terminator
| 5 PRIME Mastermix | 10uL | x3 | 30uL |
| Forward Primer | 0.5uL | x3 | 1.5uL |
| Reverse Primer | 0.5uL | x3 | 1.5uL |
| Template | 14uL | x3 | 42uL |
12th September
- Isolated genomic DNA from 8 cultures of W303a yeast cells
- Protocol from Wiley's Current Protocols in Molecular Biology
- Labeled templates 1,2,3,4 and 1a,2a,3a,4a
- Ran gel of PCR from 11th September
- 1% agarose
- 150V
- 38 mins
- Poor results
15th September
- PCR PGK Promotor
- Finnzymes Phusion High Fidelity DNA Polymerase
- F-530, 20V (2V/uL)
- Finnzymes Phusion High Fidelity DNA Polymerase
| 5x Phusion HF Buffer | 10uL | x3 | 30uL |
| 10mM dNTPs | 1uL | x3 | 3uL |
| Primer A(Forward) | 1uL | x3 | 3uL |
| Primer B(Reverse) | 1uL | x3 | 3uL |
| Template 1 | 10uL | x3 | 30uL |
| Phusions DNA polymerase | 0.5uL | x3 | 1.5uL |
| H2O | 26.5uL | x3 | 79.5uL |
18th September
- Ran reaction mentioned on 15th September
- Extracted DNA from gel from 8th September (PGK Terminator)
| Tube | Gel(g) |
| 1 | 0.332 |
| 2 | 0.278 |
| 3 | 0.307 |
| 4 | 0.349 |
| 5 | 0.385 |
19th September
- Gel of PGK Promotor from 15th September has no DNA present
23rd September
- PCR: Fus1 Downstream
- EPICENTRE Bioetechnologies - MasterAmp(TM) Taq DNA Polymerase
- Per 50uL of reaction,
| MasterAmp Taq 10x PCR Buffer | 5uL |
| 1mM dNTPs | 1uL |
| Primer 1 | 0.5uL |
| Primer 2 | 0.5uL |
| 25mM MgCl2 | 2uL |
| Taq DNA Polymerase | 0.25uL |
| Template 2 | 20uL |
| Water | 20.75uL |
- PCR Settings:
- 4mins, 94 degree celcius
- 30s, 94 degree celcius
- 30s, 5 degrees below primer melting temperature
- 1 min, 72 degree celcius -- to step 2 -- 30x
- 7 min, 72 degree celcius
24th September
- Ran gel of Fus1 Downstream from 23rd September
- Result: No DNA present on gel
25th September
- PCR: Fus1 Upstream
| Mastermix | 8.25uL | x3 | 24.75uL |
| Forward Primer | 0.5uL | x3 | 1.5uL |
| Reverse Primer | 0.5uL | x3 | 1.5uL |
| Template 3 | 20uL | x3 | 60uL |
| Water | 20.75uL | x3 | 62.25uL |
- same protocol as 23rd September
- The master mix contains the buffer, dNTPs, MgCl, and Taq
- Template 3 (3 reactions run)
30th September
- PCR: Fus1 Upstream
| Mastermix | 8.25uL | x3 | 24.75uL |
| Forward Primer | 0.5uL | x3 | 1.5uL |
| Reverse Primer | 0.5uL | x3 | 1.5uL |
| Template 3 | 20uL | x3 | 60uL |
| Water | 20.75uL | x3 | 62.25uL |
- same protocol as 23rd September
- Template 4 and 1a (3 reactions each)
- Gel: 1% agarose, 150V, 35 minutes -> poor results
- lanes 2,3 -> faint smear
1st October
- PCR: Ste2
| Mastermix | 8.25uL | x3 | 24.75uL |
| Forward Primer | 0.5uL | x3 | 1.5uL |
| Reverse Primer | 0.5uL | x3 | 1.5uL |
| Template 3 | 20uL | x3 | 60uL |
| Water | 20.75uL | x3 | 62.25uL |
- same protocol as 23rd September
- Templates 2a, 3a, 4a used (3 reactions each)
3rd October
- Ran Ste2 gel from 1st October
8th October
- PCR: PGK Terminator
| PCR Buffer | 5uL |
| 10mM dNTPs | 1uL |
| Forward Primer | 1uL |
| Reverse Primer | 1uL |
| MgCl2 | 5uL |
| Taq DNA Polymerase | 0.25uL |
| Template | 25uL |
| Water | 11.75uL |
- same protocol as 23rd September
- Use DNA extracted from gel on 18th September (5 reactions)
- Also extracted DNA from gel from 30th September (Fus1 Upstream)
9th October
- PCR: Ste2
- Protocol is the same as 23rd September
- Template used is product from 1st October (9 reactions total)
- PCR: Fus1 Upstream
- Protocal matches 23rd September
- Template used was DNA extracted from the gel from the 8th October, which came from the PCR run on 30th September (5 reactions total)
- Ran Ste2 gel from today
- Ran PGK terminator gel from yesterday
12th October
- PCR: Fus1 (3 reactions each)
- Template is products from 9th October
13th October
- Ran gel of PCR with Fus1
- Used 25uL or product, 5uL of loading dye
14th October
- Fus1 PCR
| MasterAmp Taq 10x PCR Buffer | 5uL |
| 1mM dNTPs | 1uL |
| Primer 1 | 0.5uL |
| Primer 2 | 0.5uL |
| 25mM MgCl2 | 5uL |
| Taq DNA Polymerase | 0.25uL |
| Template | 25uL |
| Water | 11.75uL |
- Template is reaction from 12th October
15th October
- PCR PGK Terminator
| 5 PRIME MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 25uL |
| Water | 4uL |
- Ran gel of Ste2 from 10/14
- Ran gel of Fus1 upstream from 10/14 (no ladder, oops)
16th October
- PCR: Fus1 Downstream, PGK Promoter
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 20uL |
| Water | 9uL |
- Tube 1: Template 4 - Fus1
- Tube 2: Template 4a - Fus1
- Tube 3: Template 4 - PGK Promoter
- Tube 4: Template 4a - PGK Promoter
- ran Gel of above
- ran Gel of PGK terminator from 10/15
- Extracted Ste2 from 10/15 and re-amplified:
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 20uL |
| Water | 9uL |
17th October
- Ran gel of Ste2 from 10/16
- Tube 1 - lane 2,3
- Tube 2 - lane 5,6
- PCR: Fus1 upstream
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 20uL |
| Water | 9uL |
- template is products from 10/14
- PCR: PGK Terminator
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 20uL |
| Water | 9uL |
- Template is products from 10/15
- Extracted Ste2(Gel from today)
18th October
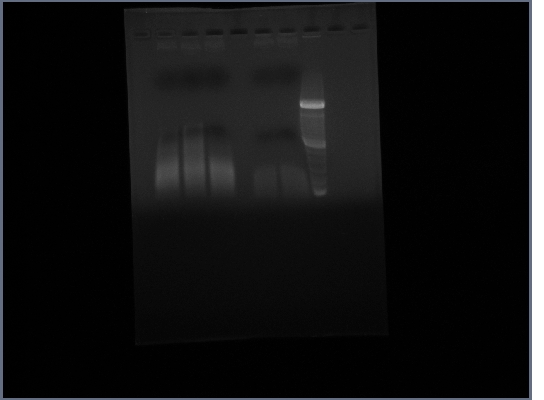
Ran gel of Fus1 upstream and PGK terminator from yesterday
20th October
- Extracting chromosomal DNA from yeast cells
- W303A - Yellow
- YPD DL
- YHP1 YPD HD - One is orange (YHP1)(Cap was removed in incubator), Other is Yellow
- Orange - YHP1 YPD HD
- Green - YHP2 YPD HD
- Pink - W303A YPA DL
- Yellow - WD303A YPD DL
21st October
- PCR: Ste2
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 20uL |
| Water | 9uL |
- 2 tubes
- Block B of black machine
- Annealing temperature: 31 degrees celcius
- Ran gel on Ste2 from today
22nd October
- New primers for biobricks are here
- Brought to standard concentration, 30uM
- Added 33.3uL of water per nmol primer
- PCR: Fus1 and PGK Terminator
| MasterMix | 20uL |
| Primer Fwd | 0.75uL |
| Primer Rev | 0.75uL |
| Template | 25uL |
| Water | 3.5uL |
- Forward and Reverse primers are new biobrick primers that arrived today
- Template is PCR product from 17th October
- A,B,C -> Fus1 Upstream -> 1,2,3
- 1,2 -> PGK Terminator -> 4,5
- Annealing temperature: 38 degrees celcius
- In freezer in yellow case
- PCR: Ste2
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 25uL |
| Water | 4uL |
- Forward primer, Reverse Primer are new primers that arrived today
- Template is PCR product from 21st October
- Two tubes in block B
- The 3 gels in the cold room do not have EtBr
23rd October
- Ran gel of Fus1 Upstream, PGK Terminator, Ste2 from yesterday
- 1% Agarose
- PCR: Fus1 Downstream, PGK Promoter
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 20uL |
| Water | 9uL |
- 4 reactions each of Fus1 Upstream(1,2,3,4) and PGK Promoter(A,B,C,D)
- Gel shows no bands.
- PCR: Fus1 Downstream, Fus1 Upstream, PGK Promoter, PGK Terminator, Ste2
- Template genomic DNA from 20th October (x4 different reactions)
- Used all Template
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 20uL |
| Water | 9uL |
- Gel shows no bands
24th October
- Ran gel of Fus1 upstream(lanes 3,4,5), PGK terminator(lanes6,7), and Ste2(lanes 8,9) from 10/22
- ladder is lane 2; 1 and 10 are nothing
- PCR of Fus1 downstream, Fus1 upstream, PGK promoter, PGK terminator, Ste2
| MasterMix | 20uL |
| Primer Fwd | 0.5uL |
| Primer Rev | 0.5uL |
| Template | 29uL |
- The templates were the rest of the genomic DNA extracts from 9/12
- Three reactions of each gene were run
- Gel from above:
- (on left)Fus1 downstream lanes 2,3,4; Fus1 upstream lanes 5,6,7; PGK promoter lanes 8,9,10;
- (on right)PGK terminator lanes2,3,10; Ste2 lanes 4,5,6; Fus1 downstream lanes 7,8,9;
25th October
- Extracted DNA from the gel from 10/24
- FUS1 upstream from the higher bands of lanes 3 and 4
- PGK terminator from the lower bands of lanes 6 and 7
- DNA ligation
| DNA | 5uL |
| buffer | 5uL |
| Re1 (Pst1) | 1uL |
| Re2 (EcoR1) | 1uL |
| Water | 37.5uL |
26th October
- Incubate for 20mins at 80 degrees celcius
| Ligation Buffer | 4uL |
| DNA Ligase | 1uL |
| DNA | 3uL |
| Plasmid | 9uL |
| Water | 3uL |
- Let sit for 5 min.
- 5uL of above mixture to competent cells
- 30 min. on ice
- Heat shock 30s (42 degrees)
- Add SOC Media (200uL)
- Incubate 60 min.(37 degrees)
- Plate 200uL
- Incubate 37 degrees celcius overnight
27th October
The transformation failed; try again with the same protocol:
- Using extracts 3 and 7 (+Ligation buffers, Ligase, Plasmid, and Water)
- Failed again.
| Home | Team | Project | Notebook | Research Articles | Parts | Protocols | Pictures |
 "
"