Team:ETH Zurich/Modeling/Overview
From 2008.igem.org
(→Overview on the modelling framework) |
(→Overview on the modelling framework) |
||
| (9 intermediate revisions not shown) | |||
| Line 11: | Line 11: | ||
==Overview on the modelling framework== | ==Overview on the modelling framework== | ||
| - | This page is meant to give an introduction to the overall modelling framework we have constructed in order to assess feasibility analysis, temporal scale details and other parameter estimations with regard of our project setup. As introduced in the [[Team:ETH_Zurich/Project/Overview|Project Overview section]], four main components can be identified in the devised mechanism. Accordingly, we divided the modeling framework in four modules that | + | This page is meant to give an introduction to the overall modelling framework we have constructed in order to assess feasibility analysis, temporal scale details and other parameter estimations with regard of our project setup. As introduced in the [[Team:ETH_Zurich/Project/Overview|Project Overview section]], four main components can be identified in the devised mechanism. Accordingly, we divided the modeling framework in four modules that address different challenges. |
<br> | <br> | ||
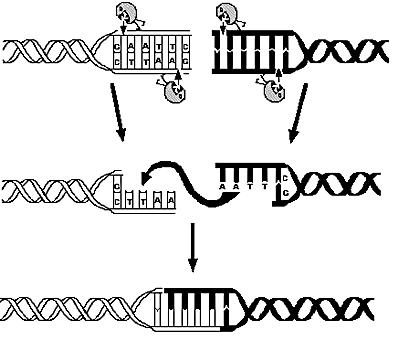
| - | The first module is concerned with the analysis of restriction enzymes and their cutting pattern on E. | + | The first module is concerned with the analysis of restriction enzymes and their cutting pattern on E.coli genome. The second module predicts the cell's response to the selection pressure and the forced genome reduction from a system point of view (that is, using a genome scale model). The third module addresses issues related to the sensitivity and setting of the chemostat mechanism. The fourth and final module presents the model of the genetic switch circuit used to control the restriction enzymes expression. |
<br> | <br> | ||
In the table below, you can find a birds-eye view on the four modules, with the most important aspects highlighted. Since we believe that a model is only useful when it answers specific and well-posed questions, this is the first aspect we report in the summary view. Second, we briefly report about the modeling method applied. Finally, we summarize the results we obtained. <br> | In the table below, you can find a birds-eye view on the four modules, with the most important aspects highlighted. Since we believe that a model is only useful when it answers specific and well-posed questions, this is the first aspect we report in the summary view. Second, we briefly report about the modeling method applied. Finally, we summarize the results we obtained. <br> | ||
| Line 30: | Line 30: | ||
* Is it possible to identify a restriction enzyme that optimizes the probability of reduced genome that retains vital strains?<br><br> | * Is it possible to identify a restriction enzyme that optimizes the probability of reduced genome that retains vital strains?<br><br> | ||
'''Method:'''<br> | '''Method:'''<br> | ||
| - | E. | + | E.coli K12 genome was digested using 713 different restriction enzymes and, using annotation information, simple statistical analysis was applied on the calculated fragments. |
<br><br> | <br><br> | ||
'''Results:''' <br> | '''Results:''' <br> | ||
| Line 45: | Line 45: | ||
* Which are the predicted quantitative differences in terms of growth rate and genome size of strains on which has been applied the selection procedure? <br><br> | * Which are the predicted quantitative differences in terms of growth rate and genome size of strains on which has been applied the selection procedure? <br><br> | ||
'''Method:''' <br> | '''Method:''' <br> | ||
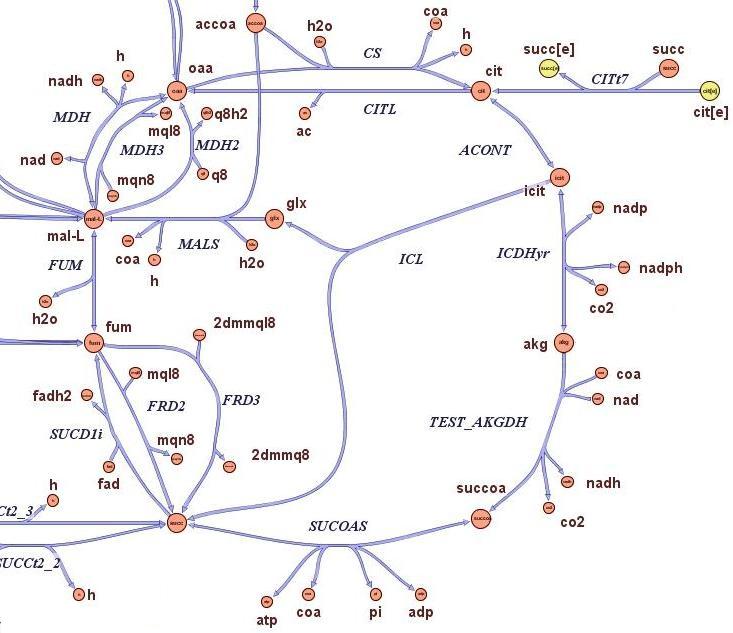
| - | The state-of-the-art genome scale model for E. | + | The state-of-the-art genome scale model for E.coli iAF1260 (1,260 genes included) was modified in order to account for thymidine auxotrophycity, thymidine uptaking limitation, genome reduction and growth on different medium. Stochastic algorithm and flux balance analysis were applied to predict growth rates.<br><br> |
'''Results:''' <br> | '''Results:''' <br> | ||
Models show that is indeed possible to select reduced genome strains using thymidine limitation. The quantification shows that the method is at the border line with the sensitivity of chemostat machinery setup for small differencies, but is effective for big reductions (from approximately 10 Kbp on). Predictions show the possibility of reducing up to 61 % of genes for a minimal medium growing strains (corresponding to 59% of chromosome size) and 73 % of genes for rich medium growing strains (corresponding to 71% of chromosome size).<br><br> | Models show that is indeed possible to select reduced genome strains using thymidine limitation. The quantification shows that the method is at the border line with the sensitivity of chemostat machinery setup for small differencies, but is effective for big reductions (from approximately 10 Kbp on). Predictions show the possibility of reducing up to 61 % of genes for a minimal medium growing strains (corresponding to 59% of chromosome size) and 73 % of genes for rich medium growing strains (corresponding to 71% of chromosome size).<br><br> | ||
| Line 55: | Line 55: | ||
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
'''Questions:''' <br> | '''Questions:''' <br> | ||
| - | * | + | * Can we identify a parameter range for substrate concentration and dilution rate in order to select for reduced strains? |
| - | * | + | * What is the sensitivity limit for selection regarding growth rate? |
| - | * | + | * How can we estimate the timing parameters between two pulses of restriction enzyme expression? |
<br><br> | <br><br> | ||
'''Method:''' <br> | '''Method:''' <br> | ||
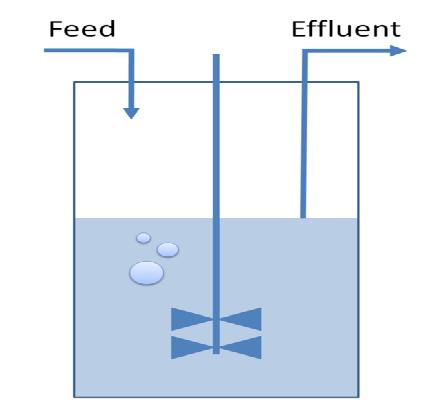
A classical chemostat model using Ordinary Differential Equations was constructed and analyzed in terms of sensitivity analysis and simulation of realistic data.<br><br> | A classical chemostat model using Ordinary Differential Equations was constructed and analyzed in terms of sensitivity analysis and simulation of realistic data.<br><br> | ||
'''Results:''' <br> | '''Results:''' <br> | ||
| - | <br><br> | + | We showed that a continuous culture is potentially a suitable environment to select for the desired organisms. Parameter ranges for the timing of pulses, dilution rate and feed concentration were identified. We developed a tool that can be refined by experimental data to predict the characteristics of the selection method.<br><br> |
</div> | </div> | ||
| valign="top" align="center" width="450"| | | valign="top" align="center" width="450"| | ||
| Line 75: | Line 75: | ||
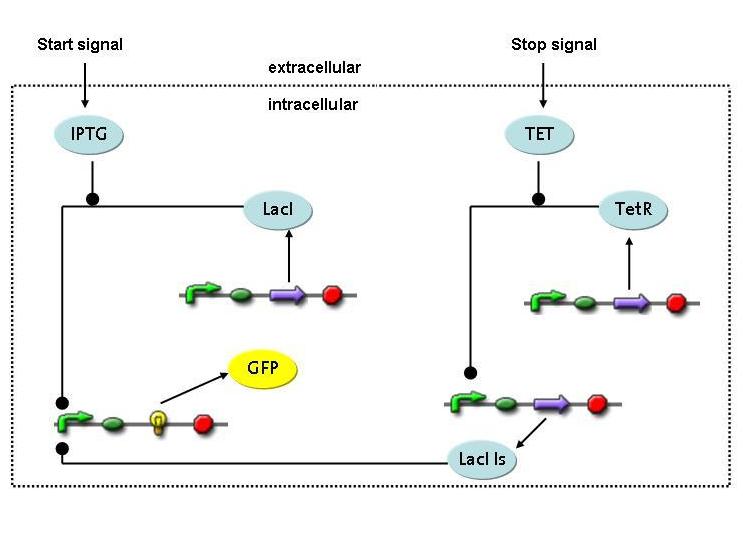
A novel transcriptional circuit able to sense an initiating and terminating signal and to control a pulse of restriction enzyme was designed and analyzed using Ordinary Differential Equations.<br> <br> | A novel transcriptional circuit able to sense an initiating and terminating signal and to control a pulse of restriction enzyme was designed and analyzed using Ordinary Differential Equations.<br> <br> | ||
'''Results:''' <br> | '''Results:''' <br> | ||
| - | The | + | The simulations show that the designed circuit is indeed able to produce a pulse-shaped response in the protein of interest which can be turned on and off by the two different inducers. Moreover the gene expression can be influenced by changing the degradation rate of the protein, for instance by tagging it, or modifying its repressors transcription rate. The pulsing frequency is mainly constrained by the time it takes for the inducers to be washed out of the medium. |
</div> | </div> | ||
|} | |} | ||
<!-- PUT THE PAGE CONTENT BEFORE THIS LINE. THANKS :) --> | <!-- PUT THE PAGE CONTENT BEFORE THIS LINE. THANKS :) --> | ||
|} | |} | ||
Latest revision as of 03:57, 30 October 2008
Overview on the modelling frameworkThis page is meant to give an introduction to the overall modelling framework we have constructed in order to assess feasibility analysis, temporal scale details and other parameter estimations with regard of our project setup. As introduced in the Project Overview section, four main components can be identified in the devised mechanism. Accordingly, we divided the modeling framework in four modules that address different challenges.
|
 "
"