Team:Harvard/Project
From 2008.igem.org
(→Make blurb shorter) |
m |
||
| (43 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
table { | table { | ||
background-color: #c4dbea; | background-color: #c4dbea; | ||
| - | font-color: | + | font-color: #cccccc; |
color:white; | color:white; | ||
} | } | ||
| Line 53: | Line 53: | ||
<!--- body here---> | <!--- body here---> | ||
| - | {|align="justify" style="background-color:#FFFFFF;text-indent: 15pt;text-align:justify" cellpadding="50" width="90%" | + | {|align="justify" style="background-color:#FFFFFF;text-indent: 15pt; font-color:#cccccc; text-align:justify" cellpadding="50" width="90%" |
| | | | ||
| - | = | + | ==BACTRICITY: Bacterial Biosensors with Electrical Output== |
| - | The metabolically versatile bacterium Shewanella oneidensis adapts to anaerobic environments by transporting electrons to its exterior, reducing a variety of environmental substrates. When grown anaerobically and provided with lactate as a carbon source, S. oneidensis transfers electrons to an electrode of a microbial fuel cell. We sought to engineer S. oneidensis to report variations in environmental conditions through changes in current production. A previous study has shown that S. oneidensis mutants deficient in the mtrB gene produce less current than the wildtype strain, and that current production in these mutants can be restored by the addition of exogenous mtrB. We attempted to control current production in mtrB knockouts by introducing mtrB on lactose, tetracycline, and heat inducible systems. These novel biosensors integrate directly with electrical circuits, paving the way for the development of automated, biological measurement and reporter systems. | + | |
| + | The metabolically versatile bacterium ''Shewanella oneidensis'' adapts to anaerobic environments by transporting electrons to its exterior, reducing a variety of environmental substrates. When grown anaerobically and provided with lactate as a carbon source, ''S. oneidensis'' transfers electrons to an electrode of a microbial fuel cell. We sought to engineer ''S. oneidensis'' to report variations in environmental conditions through changes in current production. A previous study has shown that ''S. oneidensis'' mutants deficient in the mtrB gene produce less current than the wildtype strain, and that current production in these mutants can be restored by the addition of exogenous mtrB. We attempted to control current production in mtrB knockouts by introducing mtrB on lactose, tetracycline, and heat inducible systems. These novel biosensors integrate directly with electrical circuits, paving the way for the development of automated, biological measurement and reporter systems. | ||
==Experimental overview== | ==Experimental overview== | ||
| + | |||
| + | We attempted to develop three inducible systems for electrical current production in ''S. oneidensis''. The first is a chemically inducible system, where LacI or TetR repression of the current-production gene expression in mtrB knock-out (KO) ''S. oneidensis'' can be alleviated by the addition of IPTG or anhydrotetracycline, respectively. Our second approach uses temperature-senstive cI which would allow for an increase in electrical output in response to heat. The third is a light-inducible system based on the 2005 UT Austin biological camera. | ||
| + | |||
| + | Using the LacI system, we attempted to interact with engineered ''S. oneidensis'' in multiple anaerobic microbial fuel cells which we built, measuring the effects of IPTG on current production in wild type and mtrB KO ''S. oneidensis'' containing plasmids expressing inducible or constitutive mtrB. | ||
| + | |||
==Results== | ==Results== | ||
| + | |||
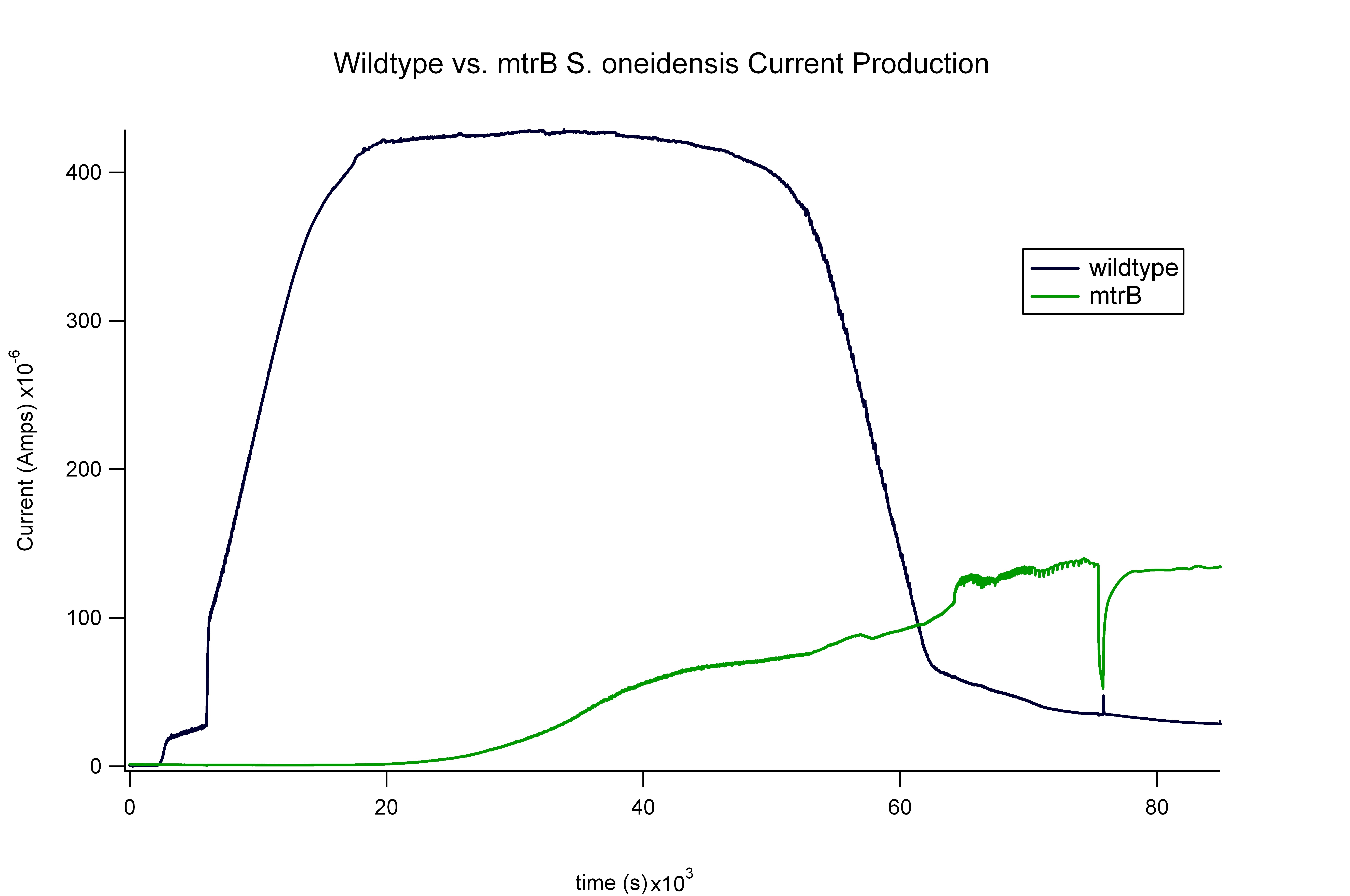
| + | ===Wildtype vs. mtrB deficient ''S. oneidensis''=== | ||
| + | |||
| + | ''Overview: By genetically manipulating S. oneidensis, it has been found that noticeable differences of current production can be detected. We wish to control current production by introducing inducible systems in these mutant strains of S. oneidensis. These differences of current production can be detected by a computer, allowing biological systems and electrical devices to be integrated.'' | ||
| + | |||
| + | The basis of our project lies in the discovery that by knocking out the mtrB gene, a current production gene of S. oneidensis, noticeable differences of current production can be detected. Therefore, this allows the current production of S. oneidensis to act more than just an on/off switch. | ||
| + | |||
| + | The first step of our project was then to test this hypothesis and also to understand the current production behavior of wildtype S. oneidensis and the mutant S. oneidensis (annotated as mtrB). Therefore, we ran a test with microbial fuel cells, one strain in each cell, and allowed the current production to be measured for approximately one day. In literature, mtrB produces approximately 20 to 25 percent of the current or wildtype strains. | ||
| + | |||
| + | |||
| + | [[Image: wt_mtrB.jpg|700px]] | ||
| + | |||
| + | |||
| + | As shown in the graph above, the results of our experiment matched those in literature. We also observed that wildtype and mtrB strains have very different current production behavior, with wildtype producing current immediately after lactate injection, and mtrB producing current much more gradually. This difference in behavior can allow for more accurate differentiation of current production between wildtype and mtrB strains when integrated with electrical devices. We wish then build on these results and build inducible systems that will allow us to control the current production. | ||
| + | |||
| + | ===Lac-inducible Strains=== | ||
| + | |||
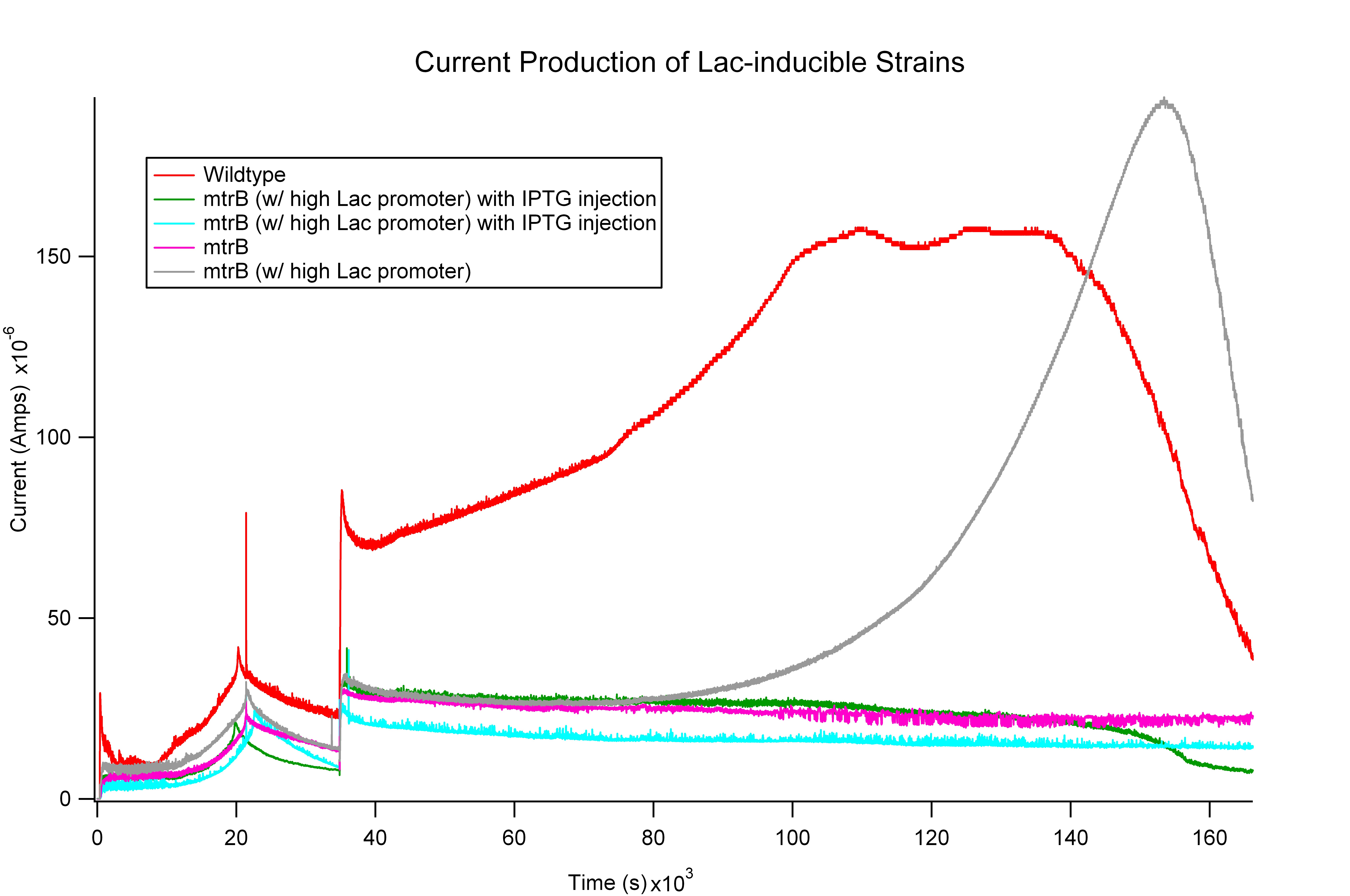
| + | ''Overview: We genetically engineered mtrB knock-out S. oneidensis MR-1 by introducing the mtrB gene on a lactose-inducible system. Specifically, we tested engineered mtrB knock-out S. oneidensis MR-1 with high lacQPI at the p15A origin. Our results show the possibility that we successfully complemented the mtrB knock-out as high levels of current were detected in one such strain.'' | ||
| + | |||
| + | Our end goal was to develop inducible systems for electrical current production in S. oneidensis MR-1. In our microbial fuel cells, we were able to test a lac-inducible system where LacI repression of the current-production gene expression in mtrB knock-out S. oneidensis MR-1 would be alleviated by the addition of IPTG. That is, the addition if IPTG to such a system would induce current production as the bacteria would begin breaking down lactate. | ||
| + | |||
| + | In this experiment, we tested the following combinations: | ||
| + | |||
| + | 1. wt S. oneidensis MR-1 + lactate | ||
| + | |||
| + | 2. mtrB knock-out S. oneidensis MR-1 with lactate | ||
| + | |||
| + | 3. mtrB (with high Lac promoter) with lactate and IPTG | ||
| + | |||
| + | 4. mtrB (with high Lac promoter) with lactate | ||
| + | |||
| + | We expected combination 1 to be highest, peaking between 100 to 200 microAmps. Combination 2 would set the baseline for the mtrB knock-out strain, usually peaking at 20 to 25 percent of wt S. oneidensis MR-1’s level. If the introduction of mtrB on a lactose-inducible system was successful, then combination 3 would also be high, roughly around the level of wt S. oneidensis MR-1. Combination 4, however, would be expected to peak around the mtrB knock-out strain’s current level as it did not receive IPTG and would not be induced. | ||
| + | |||
| + | [[ Image: Lacinducible.jpg | 700px]] | ||
| + | |||
| + | As expected, wt S. oneidensis MR-1 and the mtrB knock-out strain reached their expected levels. Interestingly, combination 4, but not combination 3, reached current levels around 200 microAmps, even greater than wt S. oneidensis MR-1’s level. That is, the engineered mtrB knock-out S. oneidensis MR-1 that did not receive IPTG resulted in elevated current production. The temporal dynamics of the curve was what we would expect from IPTG induction. That is, a delay would be observed as IPTG induction took place turning on the promoter relative to the non-inducible wt S. oneidensis MR-1. | ||
| + | |||
| + | A possible, and exciting, explanation for this observance may stem from the fact that the repressor used in our engineered strain had an LVA tag which marks it for degradation. As the experiment progressed, the repressor proteins in combination 4 may have degraded, which allowed for lactate breakdown and thus current production. The implication is that if the current production observed is due to the degradation of the repressor protein, then we successfully complemented the mtrB knock-out. We must note that if this were the case, then we should have seen an increase in all strains of the engineered mtrB knock-out if the repressor protein was being degraded. It may be that we would have observed this increase if we had run the experiment for a longer period. Future experiments may shed more light on this observance. | ||
| + | |||
| + | ===Co-Culture Experiment=== | ||
| + | |||
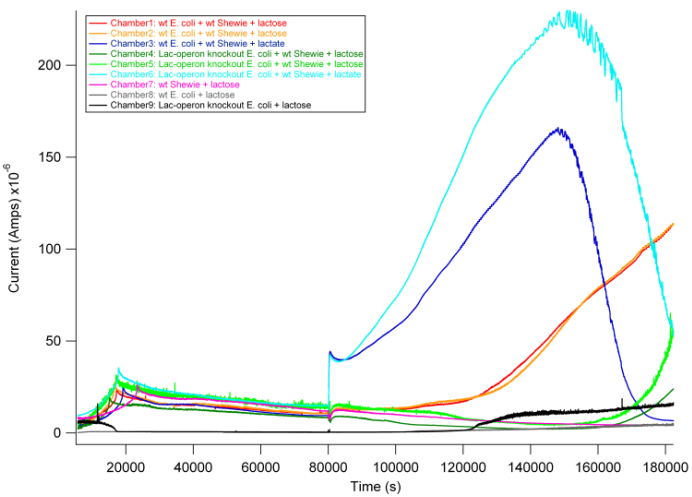
| + | ''Overview: One possible system for achieving inducible current could be to couple a inducible-lacZ system in E. coli to current production by wildtype S. oneidensis MR1 through the conversion of lactose to lactate. This leaves open the possibility of using a pre-existing E. coli lacZ reporter or creating our own. | ||
| + | '' | ||
| + | |||
| + | In addition to genetically engineering S. oneidensis MR-1 to respond to chemicals and heat, we also sought to take advantage of its natural metabolic pathway of breaking down lactate to produce current. From our wildtype versus mtrB knockout experiments, we found that feeding S. oneidensis MR-1 lactate led to significant current production almost instantly. In wildtype S. oneidensis MR-1, the current production would increase and stay elevated for a period of twelve hours. This result occurred consistently, thus we sought to use controlled lactate release as a way to control current output. | ||
| + | |||
| + | We also knew that wildtype E. coli takes lactose and breaks down lactate. Thus, we hypothesized that it might be possible to couple E. coli’s lactate production with S. oneidensis MR-1’s lactate breakdown to produce current. In addition, a great deal of genetic engineering has already been done on E. coli and the up-regulation or down-regulation of its Lac operon. This means that instead of genetically modifying S. oneidensis MR-1 to respond to chemicals or heat, we can instead take advantage of the vast library of E. coli genetics and couple genetically engineered E. coli with wildtype S. oneidensis MR-1. | ||
| + | |||
| + | In this experiment, we tested different combinations of wildtype E. coli MG1655, S. oneidensis MR-1, and Lac-operon knockout E. coli MC4100 as follows: | ||
| + | |||
| + | 1. wt E. coli (MG1655) + wt S. oneidensis (MR1) + lactose | ||
| + | |||
| + | 2. wt E. coli (MG1655) + wt S. oneidensis (MR1) + lactate pos. control | ||
| + | |||
| + | 3. Lac-operon knockout E. coli (MC4100) + wt S. oneidensis (MR1) + lactose | ||
| + | |||
| + | 4. Lac-operon knockout E. coli (MC4100) + wt S. oneidensis (MR1) + lactate pos. control | ||
| + | |||
| + | 5. wt S. oneidensis MR-1 + lactose neg. control | ||
| + | |||
| + | 6. wt E. coli (MG1655) + lactose neg. control | ||
| + | |||
| + | 7. Lac-operon knockout E. coli (MC4100) + lactose neg. control | ||
| + | |||
| + | Based on previous experiments, we would expect current production in combinations 2 and 4 as they both have wildtype S. oneidensis MR-1 and receive lactate. In addition, however, we would expect combination 1 to also produce current. As described above, wt E. coli would break down lactose into lactate, and S. oneidensis MR-1 would break down lactate to produce current. | ||
| + | |||
| + | [[ Image: picture 5.png | 750px ]] | ||
| + | |||
| + | From the data above, we found that combination 1 did indeed produce current with a delay relative to the positive control. The delay can be attributed to the time it takes for E. coli to break down lactose into lactate, thus adding an extra step in the carbon source to current production pathway compared to our positive controls. These results are exciting in that they show a possibility of taking advantage of this cooperative effort to achieve inducible current. | ||
| + | |||
==Future directions== | ==Future directions== | ||
| + | Our work with creating a system of inducible electrical output in ''S. oneidensis'' has laid the foundations for many different exciting avenues of further inquiry which look to take advantage of a bacteria-computer interface that combines the amazing sensitivity and adaptability of bacteria with the speed and analytical abilities of electricity and computers. | ||
| + | |||
| + | Using the same principles underlying the lac system, the [https://2006.igem.org/University_of_Edinburgh_2006 arsenic biosensor] developed by the University of Edinburgh iGEM 2006 team could be introduced into ''S. oneidensis'', allowing for the coupling of arsenic sensing to an electrical output, a form of a data which is easier to automate and transmit. This could be further extended to other chemical sensing systems, such as the [https://2007.igem.org/Brown lead sensor] created by the Brown iGEM 2007 team and the [https://2007.igem.org/MIT mercury sensor] made by the MIT iGEM 2007 team, resulting ultimately in an array of different strains of ''S. oneidensis'' which all respond to the presence of different chemicals with an electrical output that can be monitored by a computer. This could theoretically allow for the remote sensing and analysis of the chemical composition of an environment over time in a cost-effective manner, making it a tool with powerful public health applications, such as monitoring water quality. | ||
| + | |||
| + | Another interesting direction would be the linking of the [https://2006.igem.org/UT_Austin_2005 light-sensing system] developed by the UT Austin iGEM team with electrical output in ''S. oneidensis''. In response to variations in light, the amount of electricity produced by ''S. oneidensis'' would change. This would allow for the intriguing possibility of not only ''S. oneidensis'' conveying information to the computer, but also the computer responding to the ''S. oneidensis''. A simple example would be that in response to a chemical input, ''S. oneidensis'' may increase its electrical output. Sensing this increase, the computer could turn on or off a light directed at the ''S. oneidensis'', modifying ''S. oneidensis'''s output, creating interesting feedback loops. This could ultimately be developed into more complex communications systems between bacteria and computers. We tried constructing this system over summer, but as the process requires making an EnvZ knockout strain of ''S. oneidensis'', we could not finish it. We did, however, make a few parts to facilitate future attempts. | ||
| + | |||
| + | The possibilities are further broadened by our observations of co-cultures of ''E. coli'' and ''S. oneidensis''. Either of the systems described above could be pursued through an alternative alternative strategy of co-cultures. For instance, an array of ''E. coli'' which respond to different chemicals by breaking down lactose into lactate could be cultured with ''S. oneidensis''. In response to an increase in lactate, ''S. oneidensis'' would begin to produce higher levels of electricity. Co-cultures could also allow for more complex bacteria-computer interactions. This strategy could enable the coupling of almost any ''E. coli'' ability to electrical output. | ||
| + | |||
| + | These future directions in which our research can be taken demonstrate some of the exciting possibilities of BACTRICITY! | ||
|} | |} | ||
| - | + | ||
<!--- end body ---> | <!--- end body ---> | ||
|} | |} | ||
Latest revision as of 05:03, 30 October 2008
|
|
 "
"