Team:University of Washington/Protocols
From 2008.igem.org
(→Double Restriction Digest for AHL Cloning) |
(→Make LB) |
||
| (18 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | <html> | ||
| + | <head> | ||
| + | <style> | ||
| + | #globalWrapper { | ||
| + | margin: 0px; | ||
| + | padding: 0px; | ||
| + | background-color:#500050; | ||
| + | } | ||
| + | #content{ | ||
| + | background:#FFdd00; | ||
| + | border-left-color:#FFdd00; | ||
| + | border-right-color:#FFdd00; | ||
| + | } | ||
| + | #footer-box { | ||
| + | border-top-color:#FFdd00; | ||
| + | border-right-color:#FFdd00; | ||
| + | border-bottom-color:#FFdd00; | ||
| + | border-left-color:#FFdd00; | ||
| + | background:#FFdd00; | ||
| + | } | ||
| + | </style> | ||
| + | </head> | ||
| + | </html> | ||
| + | |||
| + | {| style="color:Gold;background-color:#500050;border: none" cellpadding="4" cellspacing="5" border="5" width="99%" align="center" | ||
| + | !align="center";style="border: none;" |[[Image:WashingtonColorSeal-21-clip.gif|50px]] | ||
| + | !align="center"; style="border: #6b0c6a outset 3px;" |[[Team:University_of_Washington|<font color="gold">Home</font>]] | ||
| + | !align="center"; style="border: #6b0c6a outset 3px;" |[[Team:University_of_Washington/Team|<font color="gold">The Team</font>]] | ||
| + | !align="center"; style="border: #6b0c6a outset 3px;" |[[Team:University_of_Washington/Project|<font color="gold">The Project</font>]] | ||
| + | !align="center"; style="border: #6b0c6a outset 3px;" |[[Team:University_of_Washington/Modeling|<font color="gold">Modeling</font>]] | ||
| + | !align="center"; style="border: #6b0c6a outset 3px;" |[[Team:University_of_Washington/Notebook|<font color="gold">Notebook</font>]] | ||
| + | !align="center"; style="border: #6b0c6a inset 3px;" |[[Team:University_of_Washington/Protocols|<font color="gold">Protocols</font>]] | ||
| + | !align="center"; style="border: #6b0c6a outset 3px;" |[[Team:University_of_Washington/Parts|<font color="gold">Parts Submitted <br>to the Registry</font>]] | ||
| + | !align="center"; style="background-color:#03943d;border: #6b0c6a outset 3px;" |[[Team:University_of_Washington/Measurement Kit|<font color="gold">Measurement Kit</font>]] | ||
| + | !align="center"; style="background-color:#c42317;border: #6b0c6a outset 3px;" |[[Team:University_of_Washington/SeToB|<font color="gold">SeToB</font>]] | ||
| + | !align="center"; style="border: #6b0c6a outset 3px;" |[[Team:University_of_Washington/Safety|<font color="gold">Safety</font>]] | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
==Glycerol Stocks== | ==Glycerol Stocks== | ||
''For the long-term storage of microbial cultures'' | ''For the long-term storage of microbial cultures'' | ||
| Line 52: | Line 92: | ||
**Primer F: 5' 40 bp your sequence + GTGTAGGCTGGAGCTGCTTC - 3' (Tm = 64C) | **Primer F: 5' 40 bp your sequence + GTGTAGGCTGGAGCTGCTTC - 3' (Tm = 64C) | ||
**Primer R: 5' 40 bp your sequence + CATATGAATATCCTCCTTAG - 3' (Tm = 54C) | **Primer R: 5' 40 bp your sequence + CATATGAATATCCTCCTTAG - 3' (Tm = 54C) | ||
| - | ''The Kan Cassette is approx. ? bp'' < | + | ''The Kan Cassette is approx. ? bp'' <br> |
''The Cm Cassette is approx. 900-1000 bp'' | ''The Cm Cassette is approx. 900-1000 bp'' | ||
*For Tet: | *For Tet: | ||
| Line 59: | Line 99: | ||
''The Tet Cassette is approx. ? bp'' | ''The Tet Cassette is approx. ? bp'' | ||
*Set up your PCR as follows: | *Set up your PCR as follows: | ||
| + | |||
| + | -34.5 uL dH20 for taq | ||
-5 uL of 10X polymerase buffer | -5 uL of 10X polymerase buffer | ||
| - | |||
| - | |||
-2.5 uL of 10 uM primer F (1:10 dilution of reconstituted stock) | -2.5 uL of 10 uM primer F (1:10 dilution of reconstituted stock) | ||
| Line 68: | Line 108: | ||
-2.5 uL of 10 uM primer R (1:10 dilution of reconstituted stock) | -2.5 uL of 10 uM primer R (1:10 dilution of reconstituted stock) | ||
| - | -2 uL | + | -2 uL of 50 mM MgCl2 (2 mM final) |
| + | |||
| + | -1 uL of 10 mM dNTPs (0.2 mM final) | ||
-0.5 uL polymerase (Invitrogen taq) | -0.5 uL polymerase (Invitrogen taq) | ||
| - | - | + | -2 uL plasmid template (or colony lysate if doing colony PCR) |
| - | <table border=1> | + | <table border=1 style="background-color:#FFdd00"> |
<tr> | <tr> | ||
<th>Segment</th><th>Cycles</th><th>Temperature</th><th>Time</th> | <th>Segment</th><th>Cycles</th><th>Temperature</th><th>Time</th> | ||
| Line 145: | Line 187: | ||
3. Add 1 μl of PfuTurbo DNA polymerase (2.5 U/μl)<br> | 3. Add 1 μl of PfuTurbo DNA polymerase (2.5 U/μl)<br> | ||
4. Do temperature cycling.<br> | 4. Do temperature cycling.<br> | ||
| - | <table border=1> | + | <table border=1 style="background-color:#FFdd00"> |
<tr> | <tr> | ||
<th>Segment</th><th>Cycles</th><th>Temperature</th><th>Time</th> | <th>Segment</th><th>Cycles</th><th>Temperature</th><th>Time</th> | ||
| Line 240: | Line 282: | ||
4. Run on gel. Controls: intact plasmid, single digest nicked plasmid, reaction without enzyme to identify any non-specific nuclease activity. | 4. Run on gel. Controls: intact plasmid, single digest nicked plasmid, reaction without enzyme to identify any non-specific nuclease activity. | ||
| - | |||
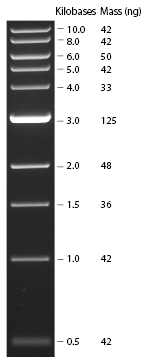
| - | [[ | + | ==Running Gel== |
| + | |||
| + | NEB 1kb Ladder<br> | ||
| + | [[Image:NEB_1kb_Ladder.jpg]] | ||
Latest revision as of 21:31, 22 January 2009

| Home | The Team | The Project | Modeling | Notebook | Protocols | Parts Submitted to the Registry | Measurement Kit | SeToB | Safety |
|---|
Contents |
Glycerol Stocks
For the long-term storage of microbial cultures
Make sure that you do not contaminate glycerol stocks
1. Grow overnight culture (1 mL) of your cells in selective media
2. Mix 750 uL of your culture with 750 uL of 40% glycerol. Immediately place at -80 C (glycerol is toxic to cells and prolonged exposure to it kills them at RT).
If you need to take from the stocks, do not thaw - just scrape some ice off of the top with a sterile loop and smear across a plate.
Double antibiotic plate
·add 20ul of the secondary antibiotic to a plate with the first antibiotic, and spread with a glass rod or inoculation loop.
Electrocompetent Cells
One Sample
This is for making one/two aliquots of electrocompetent cells
1. Grow overnight culture of your cells
2. Back-dilute 1:50 into 3 mLs (so 60 uL), put on rotator in 37 degree incubator for 3 hours (put water on ice during this time)
3. Pellet in epi tube, top speed for ~20-30 sec
4. Remove supernatant. Wash cells 3X in cold water 1 mL each.
5. Resuspend in 40 uL cold water, add DNA, electroporate
Electrocompetent DY331 for Lambda Red Recombineering
modified from the preceding protocol 07-25
1. O.N. culture in yeast incubator at 30 C
2. Back-dilute 1:50 into several 4 mL TSY broth cultures (so 80 uL), put in rotator in yeast incubator at 30 C for at least 3 hrs. Chill sterile dH20 on ice and allow the ice to melt to a slurry during the incubation.
3. OPTIONAL: Confirm OD between 0.4-0.6 before proceeding.
4. Transfer tubes to a 42 C water bath and incubate for 15 minutes. For the north water bath in the lab, this is approximately 4.2 on the temperature control knob. The increased temperature will induce Lambda red genes. Use a rotator if possible, otherwise, shake by hand every few minutes.
5. Chill and swirl in slurry for 15 minutes.
6. Divide each broth culture into three epis (or cryo-tubes for glycerol stocks). Pellet at top speed for ~20-30 sec. Wash cells 3X with 1 mL volumes of ice-cold sterile dH20.
7. Resuspend in 40 uL dH20 for immediate transformation. Alternatively, resuspend in 28 uL dH20 + 12 uL 40% glycerol and freeze -80 C immediately.
Lambda Red Recombination
This is for site-directed mutagenesis of chromosomes or large plasmids
1. Amplify antibiotic resistance cassette with primers that are homologous to cassette and your region of interest.
- For Kan or Cm:
- Primer F: 5' 40 bp your sequence + GTGTAGGCTGGAGCTGCTTC - 3' (Tm = 64C)
- Primer R: 5' 40 bp your sequence + CATATGAATATCCTCCTTAG - 3' (Tm = 54C)
The Kan Cassette is approx. ? bp
The Cm Cassette is approx. 900-1000 bp
- For Tet:
- Primer F: 5' 40 bp your sequence + TTAAGAACCCACTTTCACA - 3'
- Primer R: 5' 40 bp your sequence + CTAAGCACTTGTCTCCTG - 3'
The Tet Cassette is approx. ? bp
- Set up your PCR as follows:
-34.5 uL dH20 for taq
-5 uL of 10X polymerase buffer
-2.5 uL of 10 uM primer F (1:10 dilution of reconstituted stock)
-2.5 uL of 10 uM primer R (1:10 dilution of reconstituted stock)
-2 uL of 50 mM MgCl2 (2 mM final)
-1 uL of 10 mM dNTPs (0.2 mM final)
-0.5 uL polymerase (Invitrogen taq)
-2 uL plasmid template (or colony lysate if doing colony PCR)
| Segment | Cycles | Temperature | Time |
|---|---|---|---|
| 1 | 1 | 95°C | 1 minute |
| 2 | 30 | 95°C | 1 minute |
| 45°C | 1 minute | ||
| 72°C | 2 minutes | ||
| 3 | 1 | 72°C | 10 minutes |
| 4 | 1 | 4°C | HOLD |
2. Run small aliquot on gel to verify.
3. Qiagen PCR purification kit, elute with water.
4. Transform 15 uL into cells that express lambda red machinery
- If these cells have machinery on pKD46 plasmid: Grow O/N culture at 30C, Sub 1:100 in LB Amp100 + 0.2% arabinose, grow at 30C until OD600 is around 0.6 to 0.8 (25mLs). Wash 2X in cold water (25 mLs, spin 7' at 7K RPM), resuspend in water (100 uL) at density high enough for electroporation.
5. Recover with SOC, plate all on selective media.
6. Streak for isolation
7. Confirm by PCR
Mini Preps
Qiagen Kit
1. 250μL P1 resuspend pellet
2. 250μL P2
3. 350μL N3
4. Centrifuge full speed 10 min.
5. Decant supernatant into filter cartridge
6. Spin 1 min.
·empty collection tube
7. Add 750μL PE wash buffer
8. Spin 1 min.
·empty collection tube
9. Dry spin 2 min.
10. Put filter cartridge in clean eppendorf tube
11. Add 50μL EB
·Centrifuge 2 min. @ 9000 rpm
QuikChange Mutagenesis
Reference: [http://www.stratagene.com/manuals/200516.pdf Stratagene QuikChange® XL Site-Directed Mutagenesis Kit Instruction Manual]
Prep:
- Design the sequences
- Design primers(>= 40% GC, boiling point >= 78C, length ~25-45 bp, mutation between 10-15 correct bases of sequence, terminate in G or C)
1. Add followings to the thin-walled tube for thermocycling
- 5 μl 10× reaction buffer
- X μl (10 ng) dsDNA template
- X μl (125 ng) oligonucleotide primer #1
- X μl (125 ng) oligonucleotide primer #2
- 1 μl dNTP mix
- 3 μl DMSO
2. Add ddH2O to a final volume of 50 μl
3. Add 1 μl of PfuTurbo DNA polymerase (2.5 U/μl)
4. Do temperature cycling.
| Segment | Cycles | Temperature | Time |
|---|---|---|---|
| 1 | 1 | 95°C | 1 minute |
| 2 | 18 | 95°C | 50 seconds |
| 60°C | 50 seconds | ||
| 68°C | 1 minute/kb of plasmid length | ||
| 3 | 1 | 68°C | 7 minutes |
5. Cool on ice ~2 mins
6. Add 1 μl Dpn1(10 U/μl)
7. Mix solution using a pipet several times, Spin in a Microcentrifuge for 1 min
8. Incubate the reactions at 37°C for 1 hour
Post:
- PRC purification
- Transformation
Sequencing
From medium- or high-copy miniprepped plasmid DNA obtained via Qiagen kit
1. Dilute primer to 1 pmole/uL (= 1 uM or a 1:10 dilution of your PCR primers)
2. Aliquot 8 uL of primer (=8 pmole) per epi tube for each sequencing reaction (1 primer/tube, so if you are doing forward and reverse reaction, two tubes). Make sure that you use the correct primers for your reaction.
3. Add 4 uL Qiagen-miniprepped plasmid DNA
4. Fill out form on UW DNA Sequencing Facility website. Make sure that you are connected to a printer for this. Use budget number: 75-1064, Box number: 355761. If you do not already have an account, you will have to make one. Use your own phone number, and Herbert's info for PI information (Box 355061, William H. Foege Building, Room N210E). Select "Plasmid DNA", give short description to each tube. Write down the number they give you (usually your initials and then some number) on the top of the epindorf tube. At the bottom, select "Rxn and analysis" and "BDSF's Choice", and NO for printing.
5. Print out two copies of the form, one on colored paper (or on white paper that you can then scribble around the edges with a highlighter or a Sharpie). Give the other copy to Ingrid.
6. Submit to sequencing on the 2nd floor of the Hitchcock building. Turn in the colored form where it says "Drop off". Place your samples in the fridge that says "Rxn and analysis", anywhere in a box that says "Template and primer mix".
Conjugation
Method #1
Bac-Bac
1. Grow overnight cultures of donor and recipient cells
2. Back dilute 1:100 in 2mL of media and grow to an OD of about .1
3. Plate out 1:1E6 dilution of donor/recipient culture on a selection plate.
Bac-Yeast
1. Grow overnight cultures of donor and recipient cells
2. Back dilute 1:100 in 2mL of media and grow to an OD of about .1
3. Plate out 1:1E4 dilution of donor/recipient culture on a selection plate.
Method #2
(This is taken from the [http://jb.asm.org/cgi/content/full/180/24/6538 Bates/Cashmore/Wilkins] paper)
Bac-Bac
1. Grow donor and recipient bacteria at 37°C for three mass doublings to about 2 × 10^8 organisms per ml.
2. Mix 0.5ml of each culture and collect on a cellulose-acetate filter (Sartorius; 0.45-µm pore size; 25-mm diameter)
3. Incubate for 1 h at 37°C on prewarmed nutrient agar.
4. Resuspend cells by vigorously agitating and plate at appropriate dilutions on media selective for transconjugants.
Bac-Yeast
(This is taken from the [http://jb.asm.org/cgi/content/full/180/24/6538 Bates/Cashmore/Wilkins] paper)
1. Grow donor bacteria and recipient yeast at 30°C for three mass doublings resuspend at a dilution of about 2 × 10^8 organisms per ml.
2. Mix 0.5ml of each culture and collect on a cellulose-acetate filter (Sartorius; 0.45-µm pore size; 25-mm diameter)
3. Incubate for 1 h at 30°C on prewarmed nutrient agar.
4. Resuspend cells by vigorously agitating and plate at appropriate dilutions on media selective for transconjugants.
Double Restriction Digest for AHL Cloning
Modified from Endy protocol specifically for AHL cloning 07-26
37.5 ul of sterile dH2O
5 ul of Buffer 2
0.5 ul of BSA
5 ul of DNA (ideally, 0.2-1 ug of mini-prep or PCR product)
1 ul of XbaI
1 ul of SpeI
1. Incubate reaction at 37°C in a microtube rack in the bacterial incubator for 2 hours
2. Heat kill enzymes at 80C for 20 min.
3. Store at -20°C.
4. Run on gel. Controls: intact plasmid, single digest nicked plasmid, reaction without enzyme to identify any non-specific nuclease activity.
 "
"