Team:Hawaii/Notebook/2008-07-21
From 2008.igem.org
(Difference between revisions)
(→Restriction Digest) |
m (→Colony PCR) |
||
| (2 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== Wetlab work == | == Wetlab work == | ||
===Colony PCR=== | ===Colony PCR=== | ||
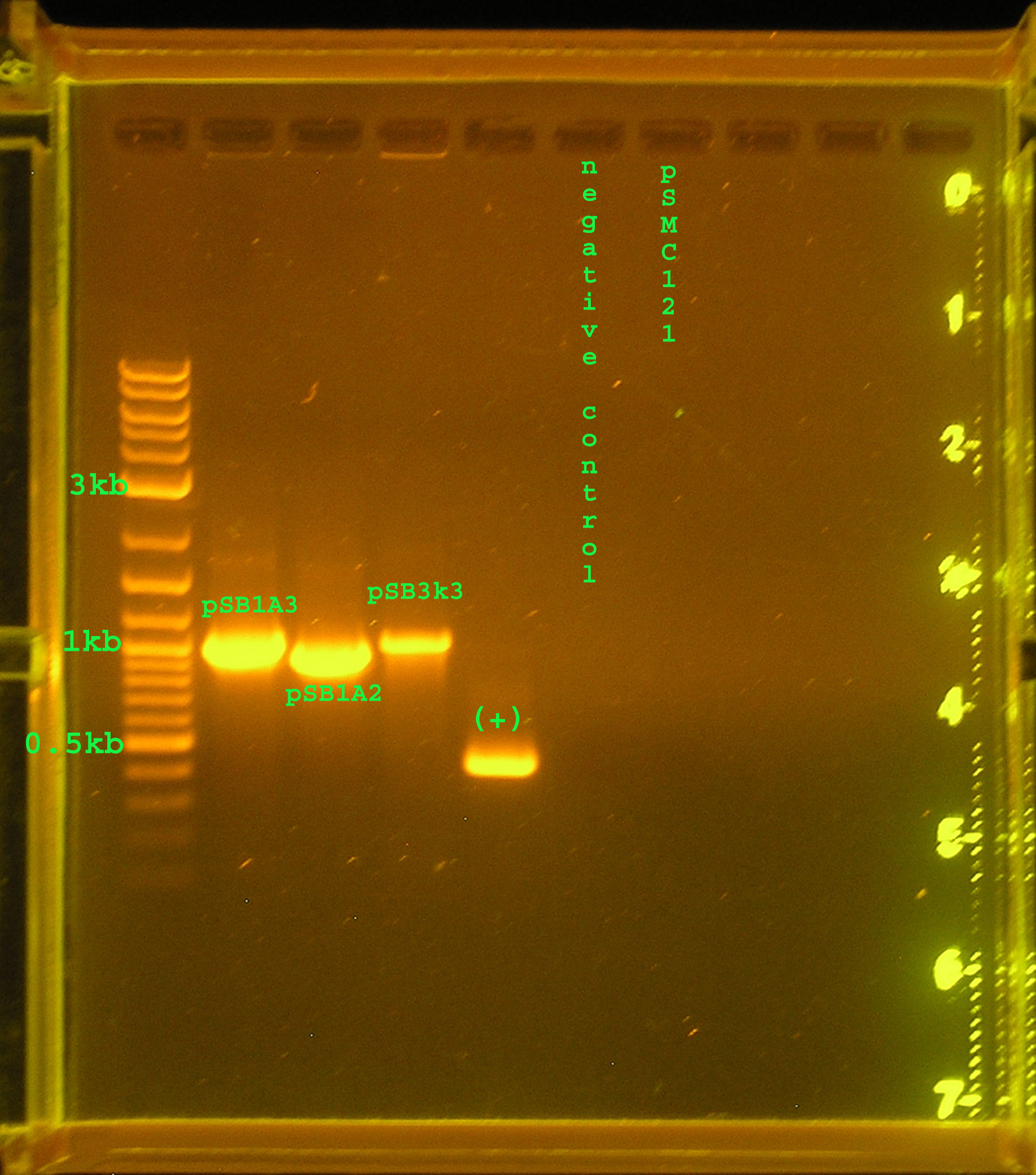
| + | [[Image:Colony_PCR_7_21.jpg|right|thumb|300px|Colony PCR: verified plasmids pSB1A3, pSB1A2, pSB3K3, and additionally verifying a sample of pSMC121 graciously donated by Dr.C.]] | ||
| + | |||
:<strong>Margaret</strong> | :<strong>Margaret</strong> | ||
:* Colony PCR of pSB1A2, pSB1A3, pSB3K3 | :* Colony PCR of pSB1A2, pSB1A3, pSB3K3 | ||
| - | + | ||
| + | <strong>Results</strong> | ||
| + | |||
| + | :*Lane 2 contains bands that are approximately 1kb, while I would expect pSB1A3 to be 991bp. Lane 3 containing pSB1A2 should be 913bp and is just above the 0.9kb mark. Lane 4 contains pSB3K3 and should be 991bp and is just below 1kb. | ||
| + | :*The positive control, UPA amplifying algal DNA is at 0.5kb as expected. | ||
| + | :*The negative control, UPA amplifying water, contains no band. | ||
| + | :*In lane 7 pSMC121, a plasmid containing the omega interposon was run, but no DNA was stained. | ||
| + | <strong> Conclusions</strong> | ||
| + | |||
| + | :*The plasmids (pSB1A3, pSB1A2, pSB3K3) were verified using VF2 and VR. I will make a glycerol stock and do a plasmid prep. | ||
| + | |||
| + | :*The absence of a a band for pSMC121 may explain the difficulty I have had in amplifying the omega region of this plasmid. I need to go get a new sample. | ||
===PCR amplification of pRL1383a parts=== | ===PCR amplification of pRL1383a parts=== | ||
| Line 25: | Line 38: | ||
===Name of Task=== | ===Name of Task=== | ||
| - | :<strong> | + | :<strong> Krystle, Margaret</strong> |
| - | :* | + | :* We figured out why some of the biobrick extractions did not transform. Some of these parts are on pSB2K3[[http://partsregistry.org/wiki/index.php?title=Part:pSB2K3]] which is a low copy until IPTG is added to the medium in which case it becomes high copy! So next time we make this, add 1mM IPTG to the media. |
| - | : | + | |
Latest revision as of 02:58, 2 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Colony PCR
- Margaret
- Colony PCR of pSB1A2, pSB1A3, pSB3K3
Results
- Lane 2 contains bands that are approximately 1kb, while I would expect pSB1A3 to be 991bp. Lane 3 containing pSB1A2 should be 913bp and is just above the 0.9kb mark. Lane 4 contains pSB3K3 and should be 991bp and is just below 1kb.
- The positive control, UPA amplifying algal DNA is at 0.5kb as expected.
- The negative control, UPA amplifying water, contains no band.
- In lane 7 pSMC121, a plasmid containing the omega interposon was run, but no DNA was stained.
Conclusions
- The plasmids (pSB1A3, pSB1A2, pSB3K3) were verified using VF2 and VR. I will make a glycerol stock and do a plasmid prep.
- The absence of a a band for pSMC121 may explain the difficulty I have had in amplifying the omega region of this plasmid. I need to go get a new sample.
PCR amplification of pRL1383a parts
- continued from Saturday, ran a gel
Restriction Digest
- Krystle
- Performed 2 restriction digests on nir, gfp, and gfp fusion brick with EcoRI and SpeI
- trial 1: digested 5ul of each plasmid prep in a 50ul reaction mixture, ran 20 ul of the digest after 3 hours
- trial 2: digested 25ul of each plasmid prep in a 50ul reaction mixture, ran 20 ul of the digest after 2 hours
Drylab Work
Name of Task
- Krystle, Margaret
- We figured out why some of the biobrick extractions did not transform. Some of these parts are on pSB2K3http://partsregistry.org/wiki/index.php?title=Part:pSB2K3 which is a low copy until IPTG is added to the medium in which case it becomes high copy! So next time we make this, add 1mM IPTG to the media.
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"