Team:Hawaii/Notebook/2008-08-12
From 2008.igem.org
(Difference between revisions)
(New page: {{Team:Hawaii/Header}} = Things we did today = == Wetlab work == ===Test of lab's RE (cont.)=== :<strong>Grace</strong> [[Image:081208enzymetest.jpg|right|thumb|200px|EtBr stained 1.2% ag...) |
(→Verified transformants) |
||
| (13 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== Wetlab work == | == Wetlab work == | ||
===Test of lab's RE (cont.)=== | ===Test of lab's RE (cont.)=== | ||
| + | |||
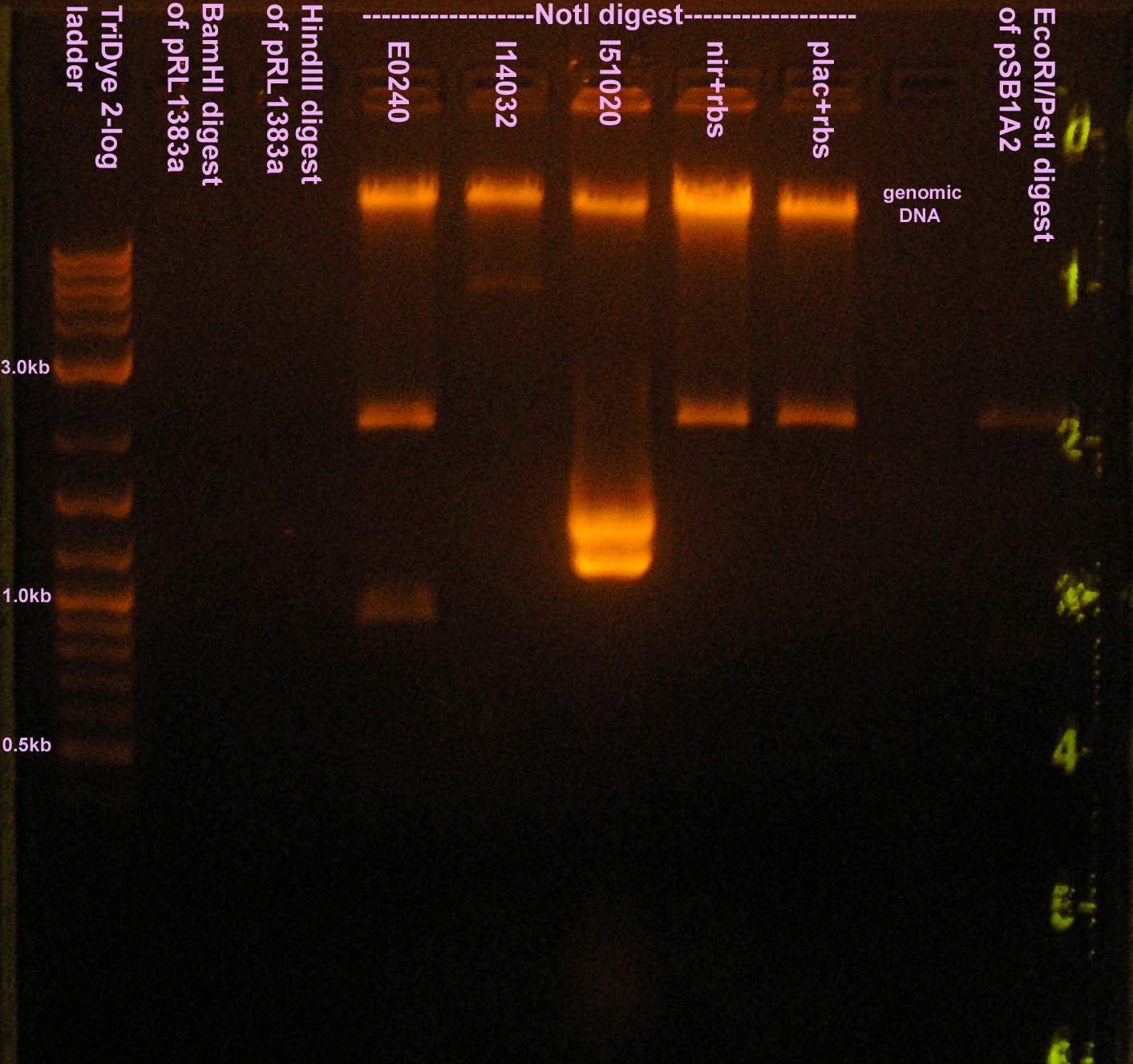
| + | [[Image:081208enzymetest.jpg|right|thumb|250px|EtBr stained 1.2% agarose gel ran at 72V for 1.5 hours. Thirty microliters of RE digest reaction were loaded into each well.]] | ||
:<strong>Grace</strong> | :<strong>Grace</strong> | ||
| - | |||
:* Ran RE digests from yesterday on a 1.2% agarose gel | :* Ran RE digests from yesterday on a 1.2% agarose gel | ||
| + | ::* BamHI and HindIII digested DNA not visualized. Too little DNA added? | ||
| + | ::* NotI works. All plasmid preps except I14032 resulted in 2 bands of the correct size. | ||
| + | :::* Note: There is genomic DNA in all preps (band that looks like fire up top) | ||
| + | |||
===Verified transformants=== | ===Verified transformants=== | ||
:<strong> Grace</strong> | :<strong> Grace</strong> | ||
| - | + | ||
{|class=wikitable border=1 align=center | {|class=wikitable border=1 align=center | ||
!Construct | !Construct | ||
!Colony forming units | !Colony forming units | ||
|- | |- | ||
| - | |GFP+B0015(tt) | + | |align=center|GFP+B0015(tt) |
| - | | | + | |align=center|1 |
|- | |- | ||
| - | |GFPf+B0015(tt) | + | |align=center|GFPf+B0015(tt) |
| - | | | + | |align=center|52 |
|- | |- | ||
| - | |slr1+GFPf | + | |align=center|slr1+GFPf |
| - | | | + | |align=center|56 |
|- | |- | ||
| - | |pilA+GFPf | + | |align=center|pilA+GFPf |
| - | | | + | |align=center|43 |
|- | |- | ||
|} | |} | ||
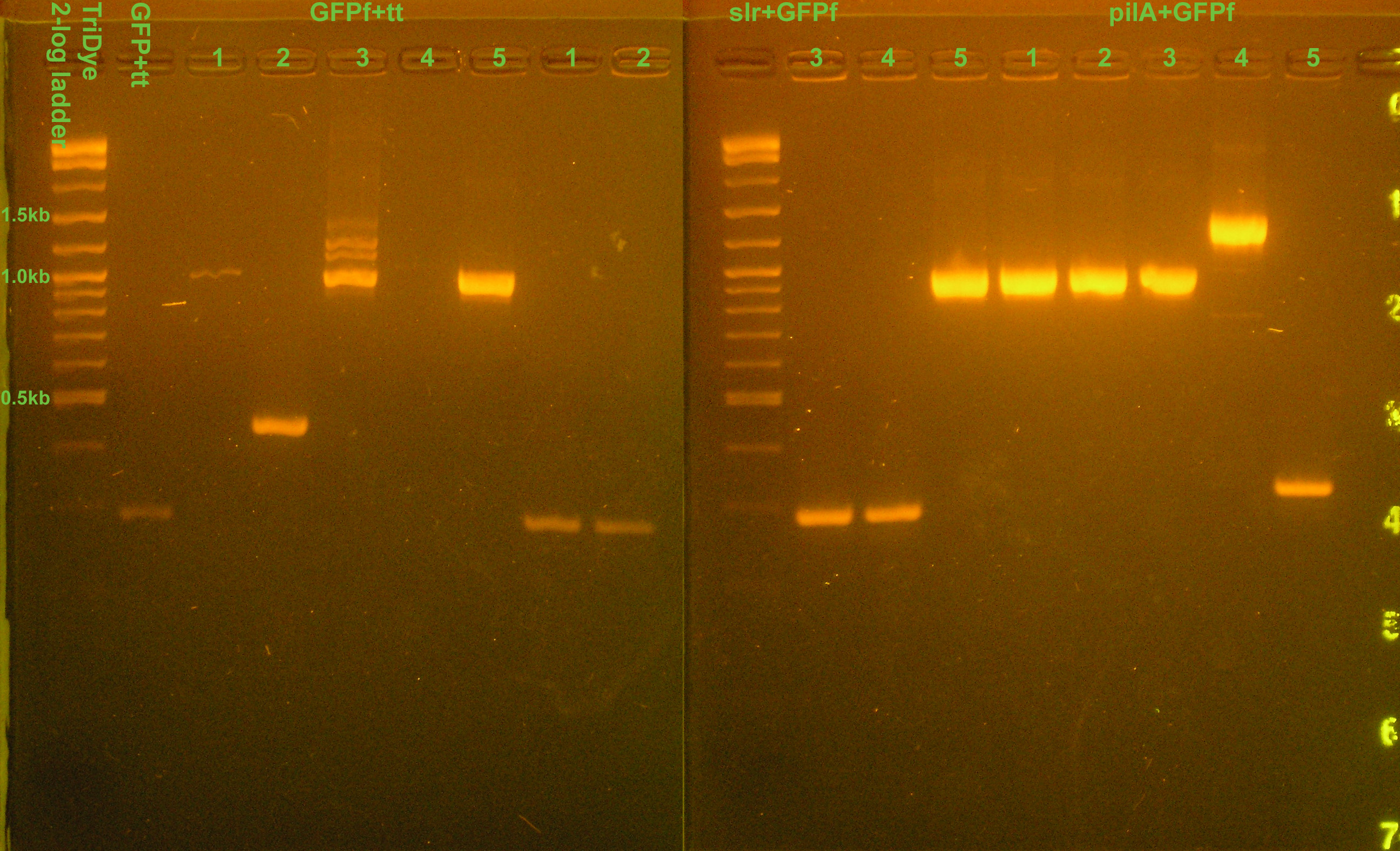
| + | [[Image:081208colonyPCR.jpg|right|thumb|300px|EtBr stained 2% agarose gel ran at 95V for 1 hour. Five microliters of PCR reaction were loaded into each well.]] | ||
:* Colony PCR of transformants to check for correct insert | :* Colony PCR of transformants to check for correct insert | ||
| + | ::* GFP+tt = unsuccessful | ||
| + | ::* GFPf+tt colonies 1, 3, 5 good | ||
| + | ::* slr1+GFPf colony 5 potentially good | ||
| + | ::* pilA+GFPf colonies 1, 2, 3, 4 potentially good | ||
| + | :::* Ligated slr, pilA with GFPf (ES digested) instead of GFPf (XP digested). Still got colonies, some which are potentially good. Hm... | ||
| + | :* Restreaked: | ||
| + | ::* GFPf+tt colony 5 | ||
| + | ::* slr1+GFPf colony 5 | ||
| + | ::* pilA+GFPf colonies 1 and 4 | ||
| + | :::* Will colony PCR again tomorrow to prep for sequencing | ||
| + | |||
===3A assembly=== | ===3A assembly=== | ||
:<strong>Grace</strong> | :<strong>Grace</strong> | ||
| - | :* Ligated p+r with g onto pSB1A2 | + | :* Ligated p+r with g or s onto pSB1A2 |
| - | ::* pnir+rbs with GFP | + | ::* pnir+rbs with GFP, slr1, pilA |
| - | ::* plac+rbs with GFP | + | ::* plac+rbs with GFP, slr, pilA |
| - | :* Transformed into DH5α | + | :* Transformed into DH5α using 5 μl ligation reaction |
| - | == | + | ===Rear ligation of signal sequences and GFPf=== |
| + | :<strong>Grace</strong> | ||
| - | + | :* Ligated slr and pilA with XbaI and PstI digested GFPf | |
| - | : | + | :* Transformed into DH5α using 5 μl ligation reaction |
| - | :* | + | ===[[Editing Team:Hawaii/Notebook/2008-08-11|Transformation=== |
| - | :* | + | <strong>Margaret</strong> |
| + | |||
| + | :*Re-streaked yesterday's transformation | ||
| + | :*Colony PCR on each of the re-streaked colonies | ||
| + | |||
| + | ===Construction of Omega Interposon=== | ||
| + | |||
| + | <strong>Margaret</strong> | ||
| + | |||
| + | :*Digested pSMC121 and pSB1A2 with SmaI | ||
| + | :*Ligated parts together (over-night ligation) | ||
| + | |||
| + | ===Plasmid Prep=== | ||
| + | <strong> Margaret </strong> | ||
| + | |||
| + | :*B0015, B0030, E0040, J33207 | ||
| + | :*Drying in hood | ||
| + | |||
| + | ===Glycerol Stock=== | ||
| + | <strong>Margaret</strong> | ||
| + | |||
| + | :*oriT (3 bottles) and I14032 (1 bottle) | ||
| + | |||
| + | ===Media Making=== | ||
| + | <strong>Margaret</strong> | ||
| + | :* Amp100 plates, Solution 3 for plasmid preps, 100mM IPTG(look in -20C with the antibiotics) | ||
| + | |||
| + | == Drylab Work == | ||
| + | |||
| + | ===Sequencing=== | ||
| + | :<strong> Grace</strong> | ||
| + | :* Downloaded sequences from CORE Hawaii. Began assembling contigs and checking sequences. | ||
= Discussion = | = Discussion = | ||
Latest revision as of 05:20, 13 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Test of lab's RE (cont.)
- Grace
- Ran RE digests from yesterday on a 1.2% agarose gel
- BamHI and HindIII digested DNA not visualized. Too little DNA added?
- NotI works. All plasmid preps except I14032 resulted in 2 bands of the correct size.
- Note: There is genomic DNA in all preps (band that looks like fire up top)
Verified transformants
- Grace
| Construct | Colony forming units |
|---|---|
| GFP+B0015(tt) | 1 |
| GFPf+B0015(tt) | 52 |
| slr1+GFPf | 56 |
| pilA+GFPf | 43 |
- Colony PCR of transformants to check for correct insert
- GFP+tt = unsuccessful
- GFPf+tt colonies 1, 3, 5 good
- slr1+GFPf colony 5 potentially good
- pilA+GFPf colonies 1, 2, 3, 4 potentially good
- Ligated slr, pilA with GFPf (ES digested) instead of GFPf (XP digested). Still got colonies, some which are potentially good. Hm...
- Restreaked:
- GFPf+tt colony 5
- slr1+GFPf colony 5
- pilA+GFPf colonies 1 and 4
- Will colony PCR again tomorrow to prep for sequencing
3A assembly
- Grace
- Ligated p+r with g or s onto pSB1A2
- pnir+rbs with GFP, slr1, pilA
- plac+rbs with GFP, slr, pilA
- Transformed into DH5α using 5 μl ligation reaction
Rear ligation of signal sequences and GFPf
- Grace
- Ligated slr and pilA with XbaI and PstI digested GFPf
- Transformed into DH5α using 5 μl ligation reaction
[[Editing Team:Hawaii/Notebook/2008-08-11|Transformation
Margaret
- Re-streaked yesterday's transformation
- Colony PCR on each of the re-streaked colonies
Construction of Omega Interposon
Margaret
- Digested pSMC121 and pSB1A2 with SmaI
- Ligated parts together (over-night ligation)
Plasmid Prep
Margaret
- B0015, B0030, E0040, J33207
- Drying in hood
Glycerol Stock
Margaret
- oriT (3 bottles) and I14032 (1 bottle)
Media Making
Margaret
- Amp100 plates, Solution 3 for plasmid preps, 100mM IPTG(look in -20C with the antibiotics)
Drylab Work
Sequencing
- Grace
- Downloaded sequences from CORE Hawaii. Began assembling contigs and checking sequences.
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"