Team:Hawaii/Notebook/2008-08-14
From 2008.igem.org
(Difference between revisions)
(sequcencing) |
m (→Restriction Digest) |
||
| (11 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
= Things we did today = | = Things we did today = | ||
== Wetlab work == | == Wetlab work == | ||
| - | === | + | ===Colony PCR=== |
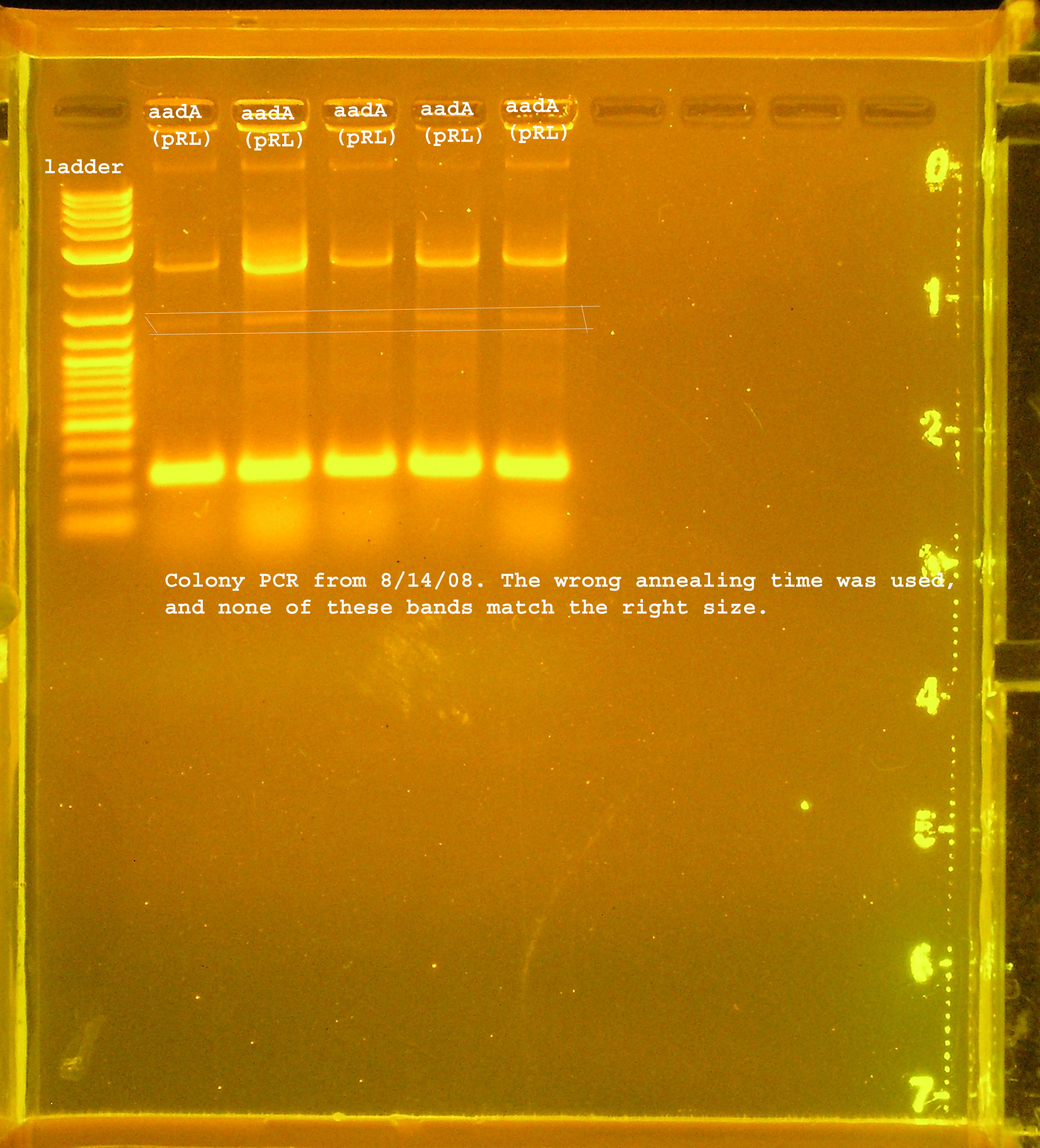
| - | :<strong> | + | [[Image: aada_verification_8_14.jpg|right|thumb|150px|PCR verification of aadA from pRL1383a using wrong conditions.]][[Image:rep_P1_omega_colony_pcr.jpg|right|thumb|150px|PCR verification of rep, P1 lytic regbion, and omega interposon ligation from 8-13]] |
| + | :<strong> Margaret </strong> | ||
| - | :* | + | :* 5 colonies of rep, P1 lytic region, and aadA(pRL1383a) region, each. pSB1A3 was used as a control. |
| - | :* | + | :* I changed the annealing time to 3min 30 seconds instead of the extension time, so the sites were not amplified properly. I ran the aadA(pRL1383a) construct on a gel and got a few bands, none of which were the right size. One of them should have been right though, so I am assuming this ligation did not work. |
| + | :*I ran another PCR verification on rep, P1, and the omega interposon and afterwards a gel. | ||
| + | |||
| + | :<strong>Krystle</strong> | ||
| + | :* '''Krystle update''' | ||
| + | ===Culture=== | ||
| + | :<strong>Margaret</strong> | ||
| + | :*Plasmid preps from [[Team:Hawaii/Notebook/2008-08-14 | yesterday]] did not yield very high quantities. I am setting up the culture for oriV1-4 and aadA(BB) 4 so I can do another plasmid prep tomorrow. | ||
| + | :<strong>Krystle</strong> | ||
| + | :* Inoculated for plasmid prep of GFPf and J04430 tomorrow. | ||
| + | ===Restriction Digest=== | ||
| + | :<strong>Margaret</strong> | ||
| + | |||
| + | :*digest of I14032 with SpeI and PstI. I want to use this to ligate to the aadA(BB) construct. | ||
| + | |||
| + | :<strong>Grace</strong> | ||
| + | |||
| + | :* Checked Krystle's RE digests from last night on a gel | ||
| + | ::* Also ran J33207 PCR product on same gel | ||
| + | ::* Excised J33207 and GFPf bands from gel | ||
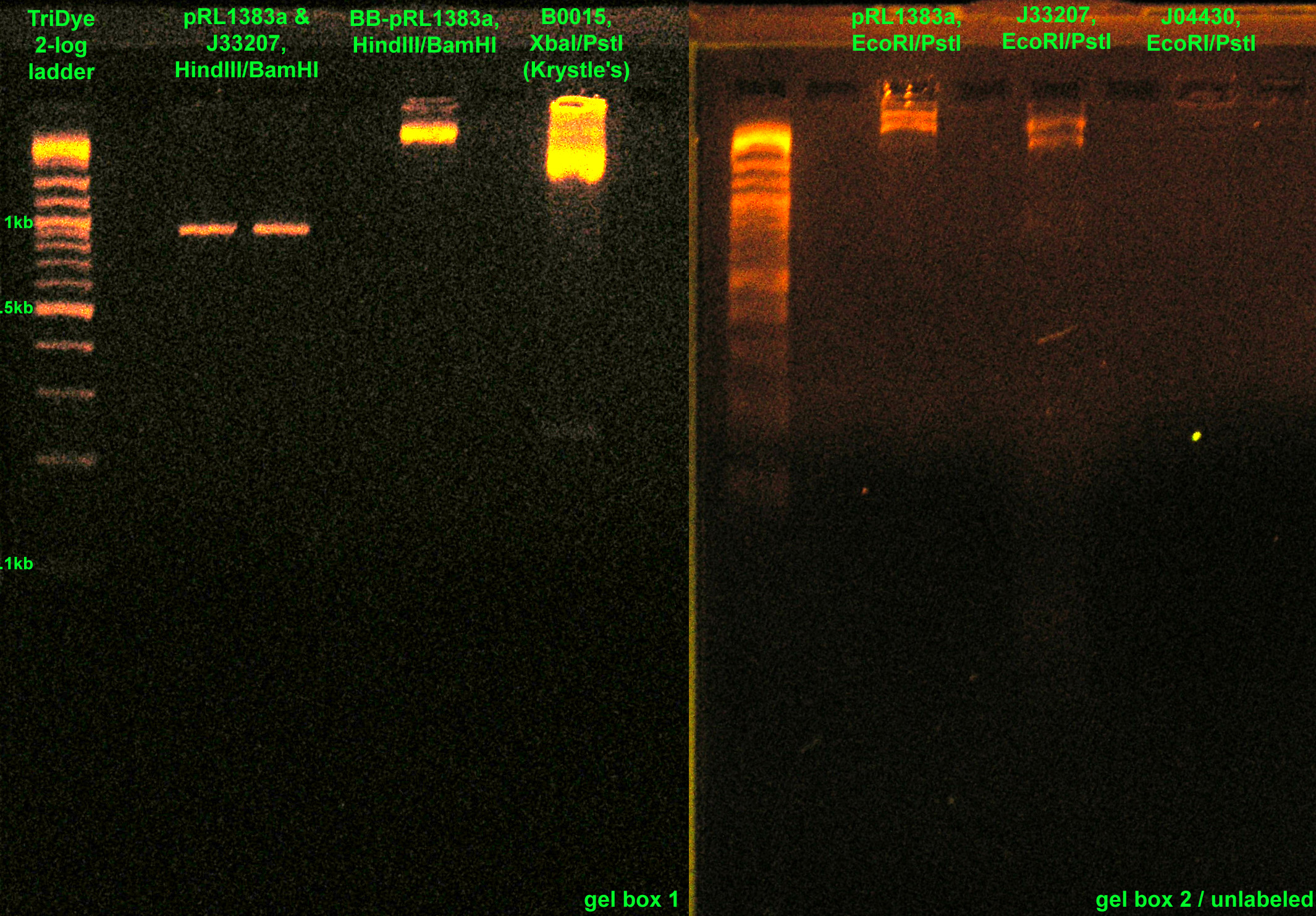
| + | :* RE digested: [[Image:081408REdigests.jpg|right|thumb|200px|EtBr stained 2% agarose gel ran at 72V for 1.5 hours. Thirty microliters of the RE digests were loaded into each well. BB-pRL1383a was digested with EcoRI/PstI. Picture is mislabeled.]] | ||
| + | ::* J33207, J04430, BB-pRL1383a with EcoRI and PstI | ||
| + | ::* pRL1383a, J33207 with HindIII and BamHI (Roche, from SC) | ||
| + | ::* BB-pRL1383a with HindIII and BamHI (from lab -20C) | ||
| + | :* Ran RE digests on a gel to purify segments of interest | ||
| + | ::* Also ran RE digested B0015 from Krystle | ||
| + | |||
| + | ===Gel extraction=== | ||
| + | :<strong>Krystle</strong> | ||
| + | |||
| + | :* Used Epoch Biolabs kit to extract J33207 and GFPf DNA from agarose gel | ||
== Drylab Work == | == Drylab Work == | ||
| Line 16: | Line 47: | ||
:* Verified sequence of oriT, inserted into pSB1A2. The sequences of oriT and the BioBrick sites are correct. | :* Verified sequence of oriT, inserted into pSB1A2. The sequences of oriT and the BioBrick sites are correct. | ||
| + | ===Oligonucleotide Design=== | ||
| + | :<strong> Margaret & Grace </strong> | ||
| + | |||
| + | :*We are having trouble with TT, RBS and Promoter ligations, so we decided to synthesize these parts. | ||
| + | :*B0016, I14032, B0030, & B0034 | ||
= Discussion = | = Discussion = | ||
| + | :* Gel box 1 is the only one that works properly. The unlabeled gel box and box #2 may cause gels to run excessively slow, bands to blur/smear, or other weird things. Amps is really low on these boxes. All covers/power sources are good. | ||
= Quote of the Day = | = Quote of the Day = | ||
| - | <blockquote>'' | + | <blockquote>''Happy chickens taste better.'' - KSGSK</blockquote> |
{{Team:Hawaii/Footer}} | {{Team:Hawaii/Footer}} | ||
__NOTOC__ | __NOTOC__ | ||
Latest revision as of 19:16, 15 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Colony PCR
File:Rep P1 omega colony pcr.jpg
PCR verification of rep, P1 lytic regbion, and omega interposon ligation from 8-13
- Margaret
- 5 colonies of rep, P1 lytic region, and aadA(pRL1383a) region, each. pSB1A3 was used as a control.
- I changed the annealing time to 3min 30 seconds instead of the extension time, so the sites were not amplified properly. I ran the aadA(pRL1383a) construct on a gel and got a few bands, none of which were the right size. One of them should have been right though, so I am assuming this ligation did not work.
- I ran another PCR verification on rep, P1, and the omega interposon and afterwards a gel.
- Krystle
- Krystle update
Culture
- Margaret
- Plasmid preps from yesterday did not yield very high quantities. I am setting up the culture for oriV1-4 and aadA(BB) 4 so I can do another plasmid prep tomorrow.
- Krystle
- Inoculated for plasmid prep of GFPf and J04430 tomorrow.
Restriction Digest
- Margaret
- digest of I14032 with SpeI and PstI. I want to use this to ligate to the aadA(BB) construct.
- Grace
- Checked Krystle's RE digests from last night on a gel
- Also ran J33207 PCR product on same gel
- Excised J33207 and GFPf bands from gel
- RE digested:
- J33207, J04430, BB-pRL1383a with EcoRI and PstI
- pRL1383a, J33207 with HindIII and BamHI (Roche, from SC)
- BB-pRL1383a with HindIII and BamHI (from lab -20C)
- Ran RE digests on a gel to purify segments of interest
- Also ran RE digested B0015 from Krystle
Gel extraction
- Krystle
- Used Epoch Biolabs kit to extract J33207 and GFPf DNA from agarose gel
Drylab Work
Sequencing
- Margaret
- Verified sequence of oriT, inserted into pSB1A2. The sequences of oriT and the BioBrick sites are correct.
Oligonucleotide Design
- Margaret & Grace
- We are having trouble with TT, RBS and Promoter ligations, so we decided to synthesize these parts.
- B0016, I14032, B0030, & B0034
Discussion
- Gel box 1 is the only one that works properly. The unlabeled gel box and box #2 may cause gels to run excessively slow, bands to blur/smear, or other weird things. Amps is really low on these boxes. All covers/power sources are good.
Quote of the Day
Happy chickens taste better. - KSGSK
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"