Team:Hawaii/Notebook/2008-08-15
From 2008.igem.org
(Difference between revisions)
(New page: {{Team:Hawaii/Header}} = Things we did today = == Wetlab work == ===Reconstruction of BB-pRl1383a=== :<strong> Grace</strong> :* PCR amplification of J33207 ::* ExoSAP'd PCR product :* R...) |
(margarets list) |
||
| (11 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
= Things we did today = | = Things we did today = | ||
== Wetlab work == | == Wetlab work == | ||
| - | ===Reconstruction of BB- | + | ===Reconstruction of BB-pRL1383a=== |
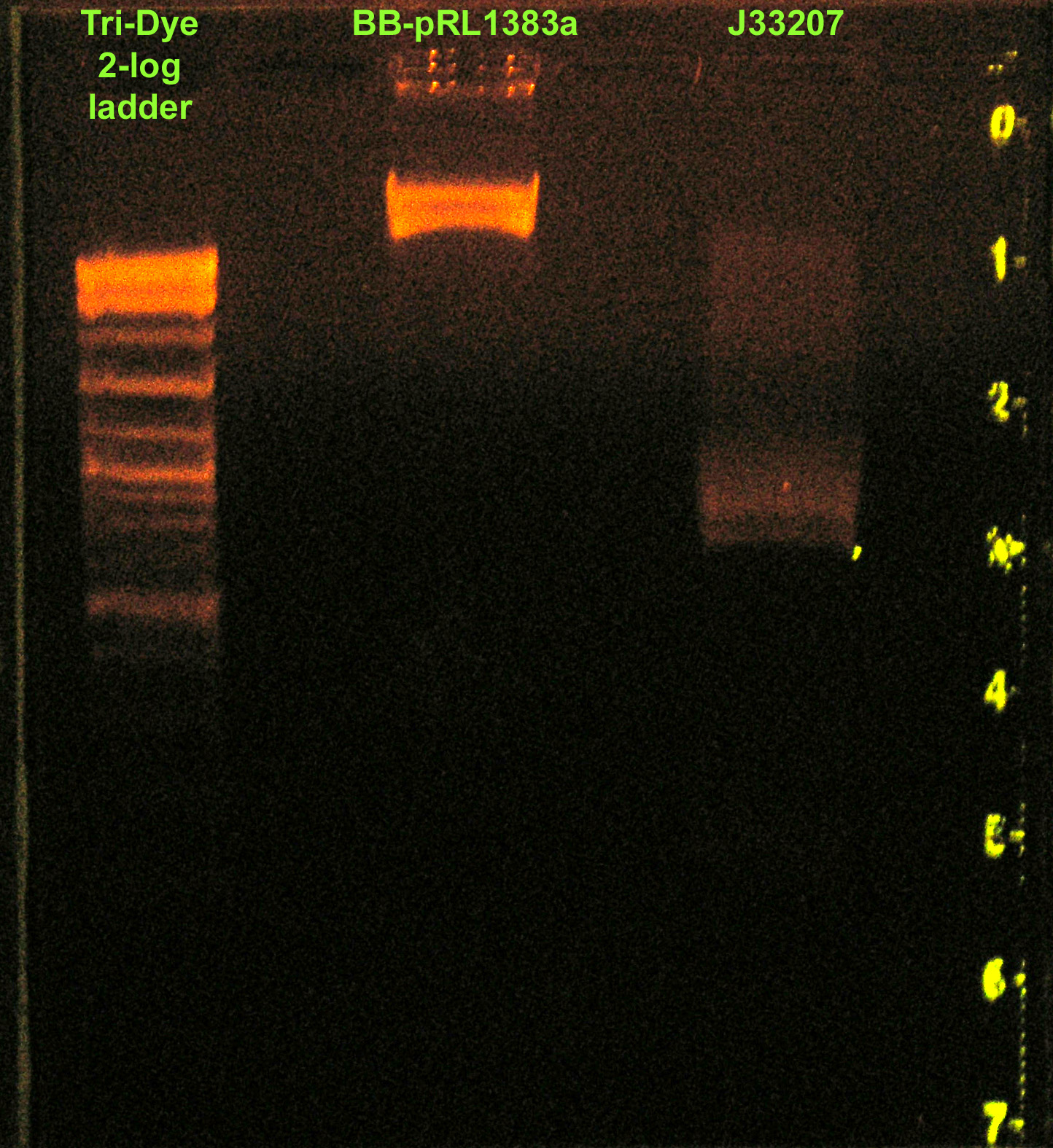
| + | [[Image:081508REdigest.jpg|right|thumb|200px|EtBr stained 1% agarose gel ran at 72V for 1.5 hours. Thirty-five microliters of RE digested BB-pRL1383a and fifty microliters of RE digested J33207 were loaded.]] | ||
:<strong> Grace</strong> | :<strong> Grace</strong> | ||
:* PCR amplification of J33207 | :* PCR amplification of J33207 | ||
::* ExoSAP'd PCR product | ::* ExoSAP'd PCR product | ||
| - | :* RE digested of J33207 and BB-pRL1383a (w/ GFP) | + | :* RE digested of J33207 and BB-pRL1383a (w/ GFP) with EcoRI and PstI |
| - | :* Ran digests on a | + | :* Ran digests on a 1% agarose gel at 72V for 1.5 hours. |
| - | : | + | ::* Ladder did not run well. Bands not resolving well. |
| - | :* | + | :* Redid PCR and RE digests with XbaI and PstI |
| - | :* | + | |
| - | == | + | ===Made 20X X-gal stock solution (20 mg/ml)=== |
| + | :<strong>Grace</strong> | ||
| - | |||
| - | |||
| - | + | ===Restriction Digest=== | |
| - | : | + | :<strong>Margaret</strong> |
| + | :*Digested J33207 (contains lacZ for blue/white screening), oriT--> these will be ligated so i can test the origin of transfer in a conjugation experiment. | ||
| + | :*Digested I14032 and the aadA(BB) which was inserted into B0030 so I can ligate these two together. | ||
| + | ===Streak to verify antibiotic resistance=== | ||
| + | :<strong>Margaret</strong> | ||
| + | |||
| + | :*On an LB plate containing Sm&Sp, the omega ligation (from 8-12). This will be a verification that the omega ligation worked. As a positive control I streaked pSMC121 which contains the omega interposon, and as a negative control I streaked pSB1A2 (in case the plates have lost their effectiveness). | ||
| + | |||
| + | == Drylab Work == | ||
| + | |||
| + | ===Editing team website=== | ||
| + | :<strong> Grace</strong> | ||
= Discussion = | = Discussion = | ||
:*DH5α does not carry ''lacI<sup>q</sup>''; therefore, IPTG is not neccessary to induce the ''lac'' promoter. | :*DH5α does not carry ''lacI<sup>q</sup>''; therefore, IPTG is not neccessary to induce the ''lac'' promoter. | ||
| + | :* IPTG is necessary for induction in DB3.1 cells. | ||
| + | |||
= Quote of the Day = | = Quote of the Day = | ||
| - | <blockquote>'' | + | <blockquote>''I'm waiting for the terminator to come in...'' - Krystle</blockquote> |
{{Team:Hawaii/Footer}} | {{Team:Hawaii/Footer}} | ||
__NOTOC__ | __NOTOC__ | ||
Latest revision as of 03:16, 16 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Reconstruction of BB-pRL1383a
- Grace
- PCR amplification of J33207
- ExoSAP'd PCR product
- RE digested of J33207 and BB-pRL1383a (w/ GFP) with EcoRI and PstI
- Ran digests on a 1% agarose gel at 72V for 1.5 hours.
- Ladder did not run well. Bands not resolving well.
- Redid PCR and RE digests with XbaI and PstI
Made 20X X-gal stock solution (20 mg/ml)
- Grace
Restriction Digest
- Margaret
- Digested J33207 (contains lacZ for blue/white screening), oriT--> these will be ligated so i can test the origin of transfer in a conjugation experiment.
- Digested I14032 and the aadA(BB) which was inserted into B0030 so I can ligate these two together.
Streak to verify antibiotic resistance
- Margaret
- On an LB plate containing Sm&Sp, the omega ligation (from 8-12). This will be a verification that the omega ligation worked. As a positive control I streaked pSMC121 which contains the omega interposon, and as a negative control I streaked pSB1A2 (in case the plates have lost their effectiveness).
Drylab Work
Editing team website
- Grace
Discussion
- DH5α does not carry lacIq; therefore, IPTG is not neccessary to induce the lac promoter.
- IPTG is necessary for induction in DB3.1 cells.
Quote of the Day
I'm waiting for the terminator to come in... - Krystle
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"