Team:Hawaii/Notebook/2008-10-10
From 2008.igem.org
(Difference between revisions)
(New page: {{Team:Hawaii/Header}} = Things we did today = == Wetlab work == ===verification of Transformants=== :<strong> Grace</strong> [[Image:101008colonypcr.jpg|right|thumb|500px|EtBr stained 2%...) |
(→Verification of Transformants) |
||
| (3 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
= Things we did today = | = Things we did today = | ||
== Wetlab work == | == Wetlab work == | ||
| - | === | + | ===Verification of Transformants=== |
:<strong> Grace</strong> | :<strong> Grace</strong> | ||
[[Image:101008colonypcr.jpg|right|thumb|500px|EtBr stained 2% agarose gel ran at 95V for 1 hour. Two microliters of PCR product were loaded into each well.]] | [[Image:101008colonypcr.jpg|right|thumb|500px|EtBr stained 2% agarose gel ran at 95V for 1 hour. Two microliters of PCR product were loaded into each well.]] | ||
| Line 29: | Line 29: | ||
|} | |} | ||
:* Colony PCR of transformants | :* Colony PCR of transformants | ||
| + | ::* nrgt #15 | ||
| + | ===Overnight RE digest=== | ||
| + | :<strong>Grace</strong> | ||
| + | :* EcoRI/PstI: | ||
| + | ::* BBpRL1383a-1 | ||
| + | ::* nrsg #6 (PCR) | ||
| + | ::* prpgt #5, 7, 11 (PCR) | ||
===Triparental Conjugation=== | ===Triparental Conjugation=== | ||
| Line 42: | Line 49: | ||
:* Placed plates in SC 37C CO<sub>2</sub> incubator until Monday | :* Placed plates in SC 37C CO<sub>2</sub> incubator until Monday | ||
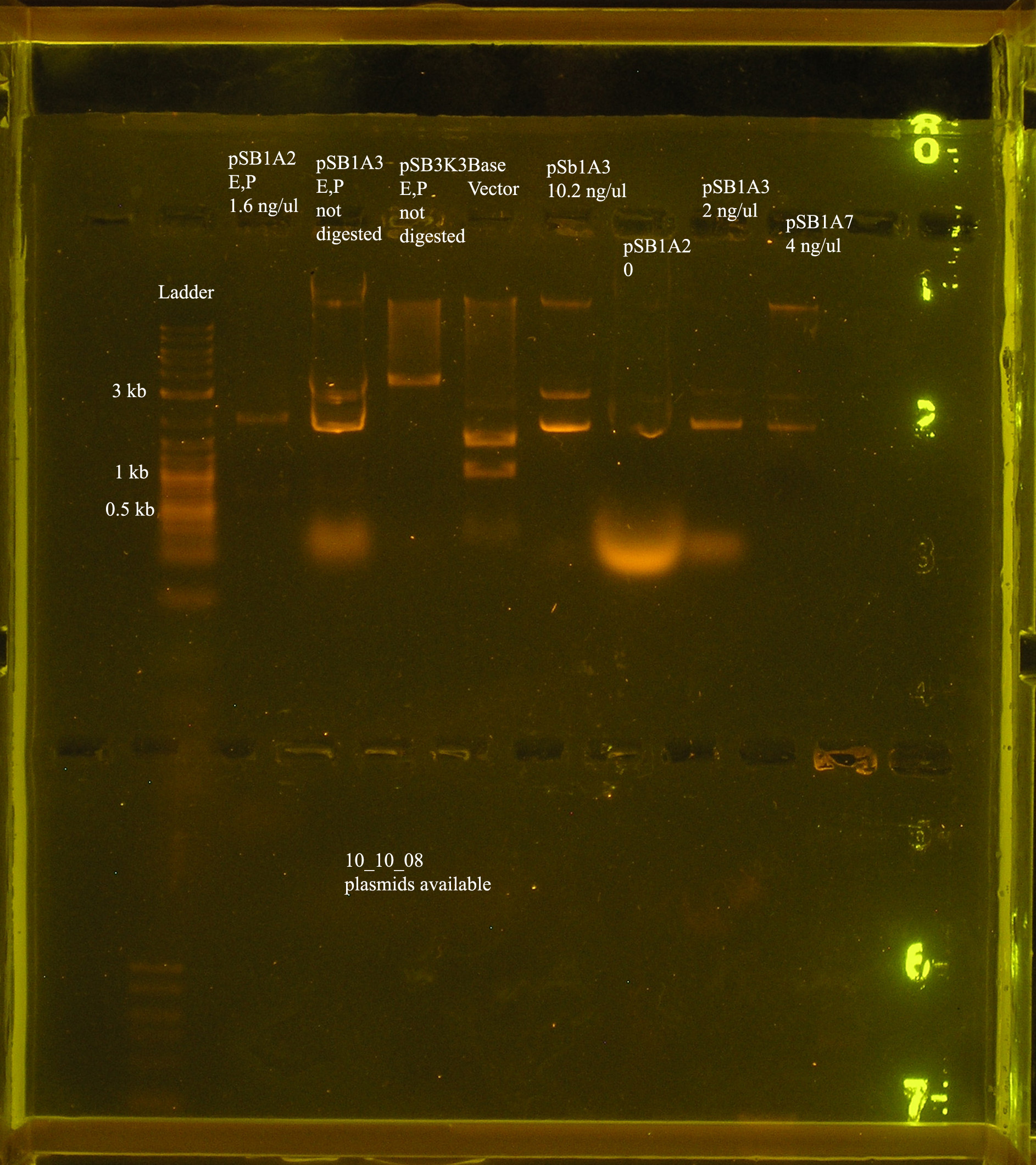
| + | ===Verification of plasmids=== | ||
| + | :<strong> MARGARET</strong> | ||
| + | [[Image:plasmid_veri_10_10.jpg|right|thumb|150px|Verification of plasmid preps and digests.]] | ||
| + | |||
| + | :*Why haven't I gotten good efficiency of transformation? I ran a gel of the plasmids i am working with and got the answer. Apparently there was a mix-up and I was using low quality plasmid. | ||
| + | :*The pSB1A3 and pSB3K3 digested and de-phosphorylated (lanes: ) were thrown out. Norman's pSB1A3 and pSB1A7 were digested with E and P today at 4:30pm. | ||
| + | :* The base vector is digested--> bands appear to be correct size. | ||
| + | |||
| + | ===Submitted sequencing=== | ||
| + | :<strong> Margaret</strong> | ||
| + | |||
| + | :*the plac/rbs/rep colony 7 construct and oriV colony 1 (from most recent transformations) were sent in for sequencing today. | ||
= Discussion = | = Discussion = | ||
Latest revision as of 03:27, 11 October 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Verification of Transformants
- Grace
File:101008colonypcr.jpg
EtBr stained 2% agarose gel ran at 95V for 1 hour. Two microliters of PCR product were loaded into each well.
| Construct | Colonies |
|---|---|
| nir+rbs+GFP+tt#1 | 100 |
| plac+rbs+GFP+tt#1 | 3 |
| nir+rbs+slr1+GFPf | 51 |
| nir+rbs+pilA+GFPf+tt | 24 |
| BBpRL1383a | lawn |
| (-) no DNA | 0 |
- Colony PCR of transformants
- nrgt #15
Overnight RE digest
- Grace
- EcoRI/PstI:
- BBpRL1383a-1
- nrsg #6 (PCR)
- prpgt #5, 7, 11 (PCR)
Triparental Conjugation
- Grace
- Began triparental conjugation between E. coli containing RP1 and BBpRL1383a -1 or -18 and Synechocystis
- RP1 OD600=0.6341
- BBpRL1383-a OD600=61.25
- BBpRL1383a-18 OD600=0.4884
- Synechocystis OD700=0.8629, OD730=0.7337
- Placed plates in SC 37C CO2 incubator until Monday
Verification of plasmids
- MARGARET
- Why haven't I gotten good efficiency of transformation? I ran a gel of the plasmids i am working with and got the answer. Apparently there was a mix-up and I was using low quality plasmid.
- The pSB1A3 and pSB3K3 digested and de-phosphorylated (lanes: ) were thrown out. Norman's pSB1A3 and pSB1A7 were digested with E and P today at 4:30pm.
- The base vector is digested--> bands appear to be correct size.
Submitted sequencing
- Margaret
- the plac/rbs/rep colony 7 construct and oriV colony 1 (from most recent transformations) were sent in for sequencing today.
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"