Team:Chiba/Calendar-Home/30 August 2008
From 2008.igem.org
(New page: >Home | Notebook 29 August 2008 <|> 31 August 2008 ==Labora...) |
(→Team:Input) |
||
| Line 14: | Line 14: | ||
<td>double</td> | <td>double</td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>6</td> | <td>6</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>10×BSA</td> | + | <td>10×BSA(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>10×NE</td> | + | <td>10×NE(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PstI</td> | + | <td>PstI(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>XbaI</td> | + | <td>XbaI(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>33.6ng/μl×30μl(1μg)</td> | <td>33.6ng/μl×30μl(1μg)</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>60</td> | <td>60</td> | ||
</tr> | </tr> | ||
| Line 61: | Line 61: | ||
<td>double</td> | <td>double</td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>4</td> | <td>4</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>10×BSA</td> | + | <td>10×BSA(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>10×NE</td> | + | <td>10×NE(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PstI</td> | + | <td>PstI(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>SpeI</td> | + | <td>SpeI(μL)</td> |
<td>4</td> | <td>4</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>75ng/μl×30μl(2μg)</td> | <td>75ng/μl×30μl(2μg)</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>60</td> | <td>60</td> | ||
</tr> | </tr> | ||
| Line 103: | Line 103: | ||
<td></td> | <td></td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>9</td> | <td>9</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>SAP</td> | + | <td>SAP(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>SAP Buffer</td> | + | <td>SAP Buffer(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>30</td> | <td>30</td> | ||
</tr> | </tr> | ||
| Line 135: | Line 135: | ||
<td>-</td><td>b.g</td> | <td>-</td><td>b.g</td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>1.4</td><td>8</td> | <td>1.4</td><td>8</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Ligase</td> | + | <td>Ligase(μL)</td> |
<td>1</td><td>1</td> | <td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Ligase Buffer</td> | + | <td>Ligase Buffer(μL)</td> |
<td>2</td><td>2</td> | <td>2</td><td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Vector</td> | + | <td>Vector(μL)</td> |
<td>1.8</td><td>1</td> | <td>1.8</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Insert</td> | + | <td>Insert(μL)</td> |
<td>3</td><td>-</td> | <td>3</td><td>-</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>10</td><td>10</td> | <td>10</td><td>10</td> | ||
</tr> | </tr> | ||
| Line 174: | Line 174: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>VF</td> | + | <td>VF(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td> | + | <td>VR2(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dNTP</td> | + | <td>dNTP(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>thermo pol buffer</td> | + | <td>thermo pol buffer(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Tag</td> | + | <td>Tag(μL)</td> |
<td>0.3</td> | <td>0.3</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>11.7</td> | <td>11.7</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>20</td> | <td>20</td> | ||
</tr> | </tr> | ||
| Line 219: | Line 219: | ||
<td>double</td> | <td>double</td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>6</td> | <td>6</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>10×BSA</td> | + | <td>10×BSA(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>10×NE</td> | + | <td>10×NE(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PstI</td> | + | <td>PstI(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>XbaI</td> | + | <td>XbaI(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>33.6ng/μl×30μl(1μg)</td> | <td>33.6ng/μl×30μl(1μg)</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>60</td> | <td>60</td> | ||
</tr> | </tr> | ||
| Line 255: | Line 255: | ||
<td></td> | <td></td> | ||
<tr> | <tr> | ||
| - | <td> | + | <td>Digested DNA Sample(μL)</td> |
<td>30</td> | <td>30</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Dye</td> | + | <td>Loading Dye(μL)</td> |
<td>6</td> | <td>6</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>36</td> | <td>36</td> | ||
</tr> | </tr> | ||
| Line 280: | Line 280: | ||
<td></td> | <td></td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>4</td> | <td>4</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Dye</td> | + | <td>Loading Dye(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>6</td> | <td>6</td> | ||
</tr> | </tr> | ||
| Line 309: | Line 309: | ||
<td>double</td> | <td>double</td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>4</td> | <td>4</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>10×BSA</td> | + | <td>10×BSA(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>10×NE</td> | + | <td>10×NE(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PstI</td> | + | <td>PstI(μL)</td> |
<td>2</td> | <td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>SpeI</td> | + | <td>SpeI(μL)</td> |
<td>4</td> | <td>4</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>75ng/μl×30μl(2.25μg)</td> | <td>75ng/μl×30μl(2.25μg)</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>60</td> | <td>60</td> | ||
</tr> | </tr> | ||
| Line 345: | Line 345: | ||
<td></td> | <td></td> | ||
<tr> | <tr> | ||
| - | <td> | + | <td>Digested DNA Sample(μL)</td> |
<td>30</td> | <td>30</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Dye</td> | + | <td>Loading Dye(μL)</td> |
<td>6</td> | <td>6</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>36</td> | <td>36</td> | ||
</tr> | </tr> | ||
| Line 371: | Line 371: | ||
<td></td> | <td></td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>9</td> | <td>9</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>SAP</td> | + | <td>SAP(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>SAP Buffer(×10)</td> | + | <td>SAP Buffer(×10)(μL)</td> |
<td>10</td> | <td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>30</td> | <td>30</td> | ||
</tr> | </tr> | ||
| Line 406: | Line 406: | ||
<td></td> | <td></td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>4</td> | <td>4</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Dye</td> | + | <td>Loading Dye(μL)</td> |
<td>1</td> | <td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>6</td> | <td>6</td> | ||
</tr> | </tr> | ||
| Line 431: | Line 431: | ||
<td>-</td><td>b.g</td> | <td>-</td><td>b.g</td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>1.4</td><td>8</td> | <td>1.4</td><td>8</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Ligase</td> | + | <td>Ligase(μL)</td> |
<td>1</td><td>1</td> | <td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Ligase Buffer</td> | + | <td>Ligase Buffer(μL)</td> |
<td>2</td><td>2</td> | <td>2</td><td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Vector</td> | + | <td>Vector(μL)</td> |
<td>1.8</td><td>1</td> | <td>1.8</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Insert</td> | + | <td>Insert(μL)</td> |
<td>3</td><td>-</td> | <td>3</td><td>-</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>10</td><td>10</td> | <td>10</td><td>10</td> | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | --> | + | -->left at rest at room temparature(25℃?本当に?) |
-->'''[[Team:Chiba/protocol/transformation|Transformation]]'''(XL10G): | -->'''[[Team:Chiba/protocol/transformation|Transformation]]'''(XL10G): | ||
-->CFU40(b.gは22) | -->CFU40(b.gは22) | ||
| - | |||
| - | |||
===Team:Communication=== | ===Team:Communication=== | ||
Revision as of 11:03, 24 October 2008
29 August 2008 <|> 31 August 2008
Contents |
Laboratory work
Team:Input
x-RBS-cI-s-p(780bp)
混ぜ表
| double | |
| dH2O(μL) | 6 |
| 10×BSA(μL) | 10 |
| 10×NE(μL) | 10 |
| PstI(μL) | 2 |
| XbaI(μL) | 2 |
| DNA(μL) | 33.6ng/μl×30μl(1μg) |
| TOTAL(μL) | 60 |
-->30μlゲル抽出
-->ゲル抽出
-->Zymo Clean
5μ抽出,うち1μlをゲルチェック-->OK,4μlはLigation用
-e-x-Ptet-s-p-(PMB1,Amp)(Insert:54bp,Vector:2079bp)
混ぜ表
| double | |
| dH2O(μL) | 4 |
| 10×BSA(μL) | 10 |
| 10×NE(μL) | 10 |
| PstI(μL) | 2 |
| SpeI(μL) | 4 |
| DNA(μL) | 75ng/μl×30μl(2μg) |
| TOTAL(μL) | 60 |
-->30μlゲル抽出
-->Zymo Clean
-->SAP
混ぜ表
| dH2O(μL) | 9 |
| DNA(μL) | 10 |
| SAP(μL) | 1 |
| SAP Buffer(μL) | 10 |
| TOTAL(μL) | 30 |
5μ抽出,うち1μlをゲルチェック
-->OK,4μlはLigation用
混ぜ表
| - | b.g | |
| dH2O(μL) | 1.4 | 8 |
| Ligase(μL) | 1 | 1 |
| Ligase Buffer(μL) | 2 | 2 |
| Vector(μL) | 1.8 | 1 |
| Insert(μL) | 3 | - |
| TOTAL(μL) | 10 | 10 |
-->Transformation(XL10G)
-->CFU40(b.gは22)
コロニーPCR(16コつつく):PCR
混ぜ表
| VF(μL) | 2 |
| VR2(μL) | 2 |
| dNTP(μL) | 2 |
| thermo pol buffer(μL) | 2 |
| Tag(μL) | 0.3 |
| dH2O(μL) | 11.7 |
| TOTAL(μL) | 20 |
-->Insertチェックしたら1つだけ正しい位置にバンドが出現。そのコロニーを2ml培養し、mini prepした。
-->Mini prep
x-RBS-cI-s-p(780bp)
混ぜ表
| double | |
| dH2O(μL) | 6 |
| 10×BSA(μL) | 10 |
| 10×NE(μL) | 10 |
| PstI(μL) | 2 |
| XbaI(μL) | 2 |
| DNA(μL) | 33.6ng/μl×30μl(1μg) |
| TOTAL(μL) | 60 |
混ぜ表
| Digested DNA Sample(μL) | 30 |
| Loading Dye(μL) | 6 |
| TOTAL(μL) | 36 |
-->Zymo Clean
-->NFW5μlで溶出
-->Gel Check
混ぜ表
| dH2O(μL) | 4 |
| DNA(μL) | 1 |
| Loading Dye(μL) | 1 |
| TOTAL(μL) | 6 |
-->Gel Check-->OK
-e-x-Ptet-s-p-(PMB1,Amp)(Insert:54bp,Vector:2079bp)
混ぜ表
| double | |
| dH2O(μL) | 4 |
| 10×BSA(μL) | 10 |
| 10×NE(μL) | 10 |
| PstI(μL) | 2 |
| SpeI(μL) | 4 |
| DNA(μL) | 75ng/μl×30μl(2.25μg) |
| TOTAL(μL) | 60 |
混ぜ表
| Digested DNA Sample(μL) | 30 |
| Loading Dye(μL) | 6 |
| TOTAL(μL) | 36 |
-->Zymo Clean
-->NFW10μlで溶出
-->SAP
混ぜ表
| dH2O(μL) | 9 |
| DNA(μL) | 10 |
| SAP(μL) | 1 |
| SAP Buffer(×10)(μL) | 10 |
| TOTAL(μL) | 30 |
-->37℃で1h、乾燥機(65℃)で15min,
-->Binding Bufferを90μl加え、VORTEX -->Zymo Clean
-->NFW5μlで抽出
混ぜ表
| dH2O(μL) | 4 |
| DNA(μL) | 1 |
| Loading Dye(μL) | 1 |
| TOTAL(μL) | 6 |
-->ゲルチェックOK
混ぜ表
| - | b.g | |
| dH2O(μL) | 1.4 | 8 |
| Ligase(μL) | 1 | 1 |
| Ligase Buffer(μL) | 2 | 2 |
| Vector(μL) | 1.8 | 1 |
| Insert(μL) | 3 | - |
| TOTAL(μL) | 10 | 10 |
-->left at rest at room temparature(25℃?本当に?) -->Transformation(XL10G):
-->CFU40(b.gは22)
Team:Communication
- Transformation
- competent cells : XL10G
- [http://partsregistry.org/Part:BBa_I9026 BBa_I9026](2007)
- [http://partsregistry.org/Part:BBa_I9030 BBa_I9030](2006)
- [http://partsregistry.org/Part:BBa_S03154 BBa_S03154](2007)
- [http://partsregistry.org/Part:BBa_S03156 BBa_S03156](2007)
- [http://partsregistry.org/Part:BBa_S03158 BBa_S03158](2007)
- [http://partsregistry.org/Part:BBa_S03160 BBa_S03160](2007)
- [http://partsregistry.org/Part:BBa_C0062 BBa_C0062](2007)
- [http://partsregistry.org/Part:BBa_C0179 BBa_C0179](2007)
- --->(31/8)Mini prep
- (29/8)--->Mini prep
- insert:C0170 + vector:J04500
- insert:C0178 + vector:J04500
- --->Digestion Test
- insert:C0170 + vector:J04500 -> Sample No.1~4
- insert:C0178 + vector:J04500 -> Sample No.5~8
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002](2007) -> Sample No.9
- Single Digestion of Sample No.1~9 -> Sample No.10~18
- Double Digestion of Sample No.1~9 -> Sample No.19~27
Sample No. Single : 10~18 Double : 19~27 Sample DNA 1 5 XbaⅠ 0.1 0.1 SpeⅠ - 0.1 Buffer 2 1 1 BSA 1 1 dH2O 6.9 2.8 TOTAL 10 10
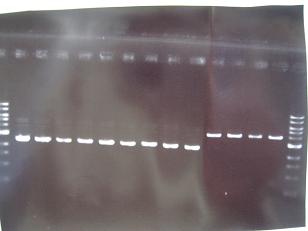
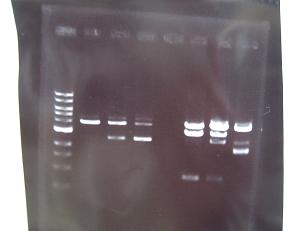
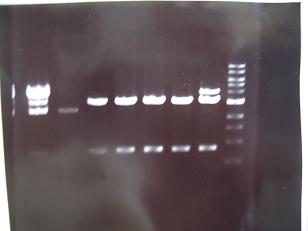
- --->Gel Check
Sample No. 1~9 10~18 19~27 Sample DNA 1 10 10 Loading Dye 1 2 2 dH2O 4 - - TOTAL 6 12 12 - From left;
- Sanple No.1~13
- -> OK
- From left;
- From left;
- Sample No.14~17,24~16
- 14 -> OK
- 15,16 -> Bad
- 17 -> None
- 24~16 -> Bad
- From left;
- Sample No.18,27,19~23
- 18,27 -> Bad
- 19~22 -> OK
- 23 -> OK??
Team:Output
- mcherry
- [http://partsregistry.org/Part:BBa_J52008 BBa_J52008](rLuc)
Sample No. [http://partsregistry.org/Part:BBa_J52008 BBa_J52008] DNA tamplate 1 rLuc_fwd 2.5 Rev primer 2.5 Thermo pol Buffer 5 dNTPmix 5 Vent pol 0.5 dH2O 34 TOTAL 50
 "
"