Team:Heidelberg/Notebook/Sensing Group/Notebook/1stweek
From 2008.igem.org
(Difference between revisions)
(→Cloning of LuxP) |
(→Cloning of LuxP) |
||
| Line 137: | Line 137: | ||
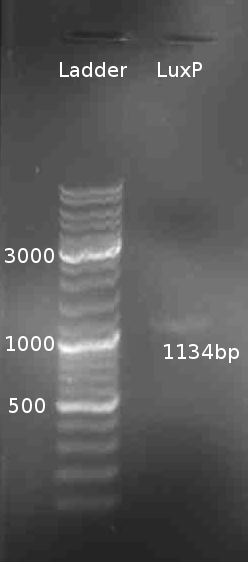
[[Image:HD_080805-LuxP_PCR.png|right|thumb|150px|LuxP colony PCR]] | [[Image:HD_080805-LuxP_PCR.png|right|thumb|150px|LuxP colony PCR]] | ||
* Gel Purification of LuxP eluted in 30 µl H<sub>2</sub>O | * Gel Purification of LuxP eluted in 30 µl H<sub>2</sub>O | ||
| + | * Digestion of LuxP with SalI and NotI of pDK48 and LuxP-Gel-Purification product: 2 µl NEB-Buffer 3 + 0.5 µl SalI/NotI + 10 µl DNA + 7 µl H<sub>2</sub>O | ||
== Tuesday, 08/05/2008 == | == Tuesday, 08/05/2008 == | ||
Revision as of 09:52, 26 October 2008


Contents |
Monday, 08/04/2008
Preparations
Preparations were done at the end of the week before
- Transformation of pDK48 and pTrc99a in E. coli DH5a (0.5 µl plasmid-DNA + 50 µl competent cells)
- Glycerol-stock of Vibrio harveyi BB120, BB886, mm30, BB178, BB125
- picked pTrc99a and pDK48 cultures and inoculated in 3 ml LB overnight
- V. harveyi cultured on LB+-Agar plate
- MiniPrep of pTrc99a and pDK48 from DHha
Cloning of LuxP
- PCR of LuxP from V. harveyi colony
- Gel Purification of LuxP eluted in 30 µl H2O
- Digestion of LuxP with SalI and NotI of pDK48 and LuxP-Gel-Purification product: 2 µl NEB-Buffer 3 + 0.5 µl SalI/NotI + 10 µl DNA + 7 µl H2O
 "
"