Team:Heidelberg/Notebook/Sensing Group/Notebook/10thweek
From 2008.igem.org
(Difference between revisions)
(→Friday, 10/10/2008) |
(→Friday, 10/10/2008) |
||
| Line 175: | Line 175: | ||
<div style="float: right; clear: none;">[[Image:HD 081011-Fusion GA Prefix Suffix PCR.png|right|thumb|150px|Prefix/Suffix PCR of GeneArt Fusion constructs]]</div> | <div style="float: right; clear: none;">[[Image:HD 081011-Fusion GA Prefix Suffix PCR.png|right|thumb|150px|Prefix/Suffix PCR of GeneArt Fusion constructs]]</div> | ||
| - | * LuxS digestion with BamHI/EcoRI to check for insert | + | * LuxS digestion with BamHI/EcoRI (NEBuffer EcoRI + BSA) to check for insert |
* LuxS mutagenesis PCR for EcoRI did not work | * LuxS mutagenesis PCR for EcoRI did not work | ||
* Prefix/Suffix PCR of GeneArt Construct | * Prefix/Suffix PCR of GeneArt Construct | ||
Revision as of 23:47, 26 October 2008


Contents |
Monday, 10/06/2008
- Mutagenesis PCR of LuxS,P with Phusion and the following programme (20 cycles)
- PCR programme: 30 sec @ 98°C || 10 sec @ 98 °C | 30 sec @ 55 °C | 30 sec @ 72 °C || 5 min @ 72 °C | 4°C
--> No PCR product
Tuesday, 10/07/2008
- Repetition of Mutagenesis PCR with Phusion and same programme, but 30 cycles
--> only LuxS2 positive --> Gelextraction
- New PCR with Turbo Pfu and iProof (18 cycles)
- 5(10) µl buffer
- 1 µl forward Primer (10 µM stock)
- 1 µl reverse Primer
- 1 µl dNTPs
- 2 µl Template
- 39(34) µl water
- 1 µl Polymerase
- 30 sec @ 95 °C ||30 sec @ 95 °C | 60 sec @ 55 °C | 30 sec @ 68 °C || 8 min @ 68 °C | 4°C hold
Wednesday, 10/08/2008
- Miniprep of LuxS
- New PCR of LuxS and LuxP with Cloned Pfu and Phusion Polymerase
- Cloned Pfu:
- 30s @ 95°C || 30s @ 95°C | 1min @ 55°C | 1min @ 68°C || 8min @ 72°C | 4min (20 cycles)
- Cloned Pfu:
- Phusion:
- 30s @ 98°C || 10s @ 98°C | 1min @ 72°C || 5min @ 72°C | 4°C (25 cycles)
- Phusion:
-->NO PCR Product
- New PCR with Taq:
- 2min @ 95°C || 30s @ 95°C | 30s @ 55°C | 1min @ 72°C || 10 min @ 72°C | 4°C (20 cycles)
-->NO PCR Product
Check Templates tomorrow, by digestion and undigested
Thursday, 10/09/2008
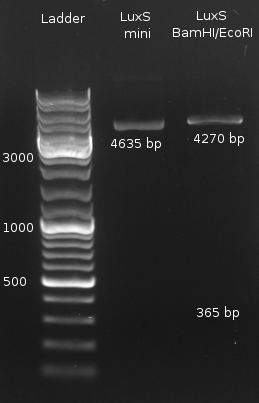
- Digestion of LuxS with (BamHI/NcoI and BamHI/EcoRI) and LuxP (NcoI) both negative
- O/N culture of LuxS
- Cloning of Tar-YFP from pDK58
- Digestion of pDK58, F1-LuxP-pDK48, F2-LuxP-pDK58 with KpnI/NotI
- Gelextraction eluted in 30 µl H2O
- Ligation of 5µl Insert with 5µl Vector 30 min @ 30°C
- Transformation into DH5a
Friday, 10/10/2008
- LuxS digestion with BamHI/EcoRI (NEBuffer EcoRI + BSA) to check for insert
- LuxS mutagenesis PCR for EcoRI did not work
- Prefix/Suffix PCR of GeneArt Construct
- PCR of LuxS1, LuxS2, LuxS3 with Phusion. Annealing Temperature Gradient 50-60°C
 "
"