Team:Heidelberg/Notebook/Sensing Group/Cloning
From 2008.igem.org
(→LuxS) |
|||
| Line 474: | Line 474: | ||
=== LuxS === | === LuxS === | ||
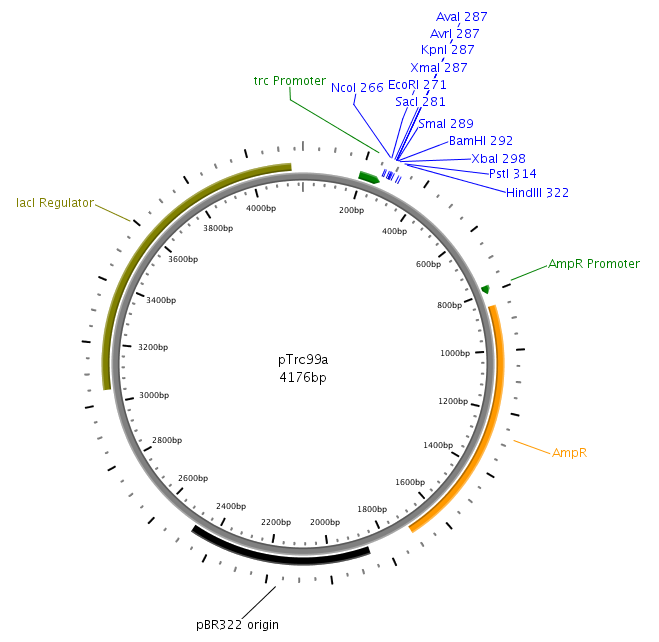
| - | LuxS will be amplified from the '' | + | LuxS will be amplified from the ''Vibrio harveyi'' genome using the following primers: LuxSa and LuxSb. The complete sequence of the used primers can be found in tableXXX. Subsequently, the product will be cloned into the plasmid pTr99alpha using the NcoI and BamHI restriction sites and then transformed into DH5alpha competent cells. |
=== LuxQ === | === LuxQ === | ||
Revision as of 18:48, 27 October 2008


Contents |
Sensing - Cloning strategy
The core part of the sensing project is the construction of the LuxQ-Tar chimeric receptor, which enables the E. coli killer bacteria to chemotactically respond to a Autoinducer 2 (AI-2) gradient and detect prey cells. The quorum-sensing system will be amplified from the V. harveyi genome while the Tar receptor is from E. coli. On one hand LuxS needs to be cloned and transformed into one cell type, in order to make them produce and secrete AI-2. On the other hand the periplasmic ligand binding domain of LuxQ will be fused with the cytoplasmic domain of Tar and cloned on one plasmid together with LuxP which is necessary for AI-2 binding. Generally, restriction sites required for cloning are introduced via the PCR primer. In silico cloning was performed in [http://serialbasics.free.fr/Serial_Cloner.html SerialCloner], Vector maps were designed with PlasMapper [1].
LuxS
LuxS will be amplified from the Vibrio harveyi genome using the following primers: LuxSa and LuxSb. The complete sequence of the used primers can be found in tableXXX. Subsequently, the product will be cloned into the plasmid pTr99alpha using the NcoI and BamHI restriction sites and then transformed into DH5alpha competent cells.
LuxQ
LuxQ will be amplied with primers LuxQa and LuxQc from the V. harveyi genome. The fragment will then be cloned into pTrc99alpha plasmid at the NcoI and BamHI site. Subsequently the product will be used as template for constructing the Fusion receptors.
LuxP
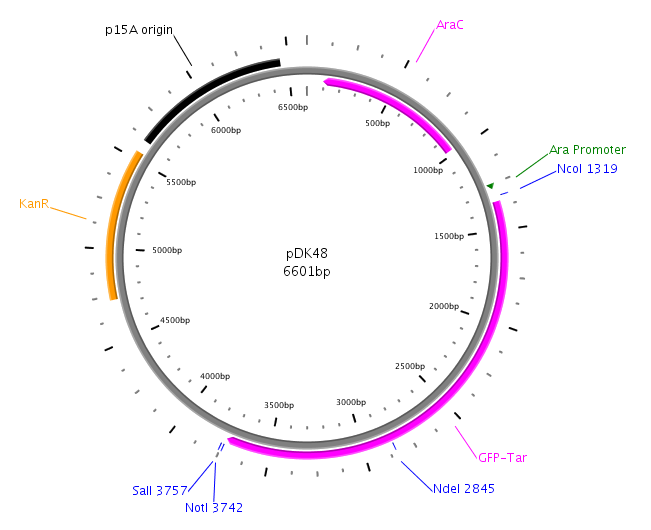
LuxP will be amplied with primers LuxPc and LuxPd from the V. harveyi genome. Then the product will be cloned into native pDK48 plasmid. After the construction of the LuxQ-Tar Fusion gene in pDK48 plasmid, LuxP will also be introduced in the pDK48 plasmid at SalI and NotI sites. The whole construct of pDK48 will be transformed into our killer cell.
Fusion receptor
A detailed description of the Tar receptor and LuxQ quorum-sensing receptor can be found in the project description. Since it is believed that the linker region (transmembrane domain TM2) is critically for signal transduction, two different receptor constructs are designed, referred to as Fusion-1 and Fusion-2. Fusion-1 contains the cytoplasmic domain of Tar and TM2 to the N-terminal part of LuxQ (TM2, periplasmic domain, TM1). Fusion-2 contains the cytoplasmic domain and TM2 of Tar and the periplasmic domain to the N-terminal end of LuxQ (periplasmic domain, TM1).
The receptor chimeras will be built in two sequential PCR reactions (Fusion PCR). In the first PCR the two single fragments containing parts of the coding sequence of LuxQ and Tar will be generated. The reverse primer for LuxQ and the forward primer for Tar part have complementary ends allowing annealing during the second PCR reaction. In addition to the amplified sequences from the first reaction the forward primer for LuxQ and the reverse primer for Tar will be added to the reaction mix after the gel purification in order to obtain the chimeric receptor sequence.
References
[1] Dong, X.; Stothard, P.; Forsythe, I. J. & Wishart, D. S., PlasMapper: a web server for drawing and auto-annotating plasmid maps, Nucleic Acids Res, Vol. 32, pp. W660-W664, 2004
 "
"