Team:Heidelberg/Notebook/Killing I/Notebook/week12
From 2008.igem.org
(Difference between revisions)
(→Phage cloning strategy two) |
(→Phage cloning strategy two) |
||
| Line 511: | Line 511: | ||
[[Image:Hd-phage-cloningtwo-ligationfragments.jpg]] | [[Image:Hd-phage-cloningtwo-ligationfragments.jpg]] | ||
| + | *lane0: DNA ladder mix | ||
| + | *lane1: purified 5.1kb band from last gel (pBluescript backbone from phage cloning strategy one (KpnI/AgeI) | ||
| + | *lane2: CmR1 digested (KpnI/SacI) | ||
| + | *lane3: CmR2 digested (KpnI/SacI) | ||
| + | *lane4: GFP1 digested (SacI/AgeI) | ||
| + | *lane5: GFP2 digested (SacI/AgeI) | ||
| + | |||
| + | *ligation of purified 5.1kb band from last gel (pBluescript backbone from phage cloning strategy one (KpnI/AgeI) with CmR1/2 and GFP1/2 | ||
| + | **30min at room temperature | ||
| + | *transformation in TOP10 | ||
==Wednesday, 10/22/08== | ==Wednesday, 10/22/08== | ||
Revision as of 12:47, 29 October 2008


| << Week 11 | Overview | Week 13 >> |
|---|
Week 12
Contents |
Monday, 10/20/08
Proceedings of phage cloning strategy one
- the fully mutated insert in pBluescript SK II was digested with XbaI/XhoI and the insert extracted from the gel
- lambda DNA was also cut with XbaI/XhoI and the backbone also extracted from the gel.
- the insert was ligated in the lambda backbone over night using standard ligation methods
Phage cloning strategy two
- Digestion with XbaI/XhoI
- should be: 3945, 2898
- Gel
- lane 1-8: new minipreps
- lane 9: old insert fully mutated
- results: the new minipreps seem to be the old unmutated pBluescript
Tuesday, 10/21/08
Proceedings of phage cloning strategy one
- the emerging plasmid consisting of our insert in tha lambda phage was transformed in E. coli by chemical transformation and in vitro packaging
- the bacterias were plated on chloramphenicol and incubated at 28 °C to ensure that the thermolabile cI (cI857) was not degraded
Phage cloning strategy two
- do again a digestion of mutagenesis pcr minipreps from 10/07/08 but with an additional restriction enzyme (EcoRI)to get a better separation on gel to cut out the backbone
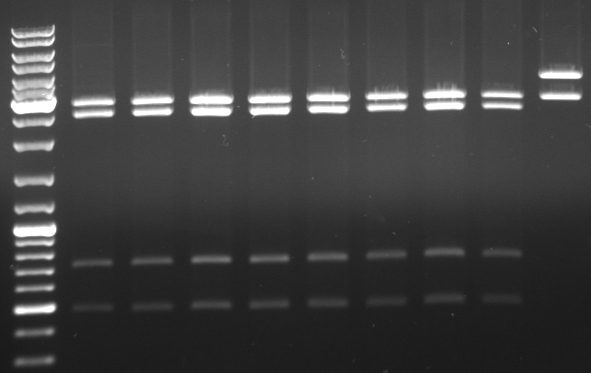
- digestion with KpnI/AgeI/EcoRI
- mutagenesis pcr succesful: 300bp, 1.4kb 5.1kb
- mutagenesis pcr not succesful: 300bp, 1.4kb, 1.7kb, 3.4kb
- -->mutagenesis pcr on the first three lanes successful
- cut out 5.1kb band and gel purification kit
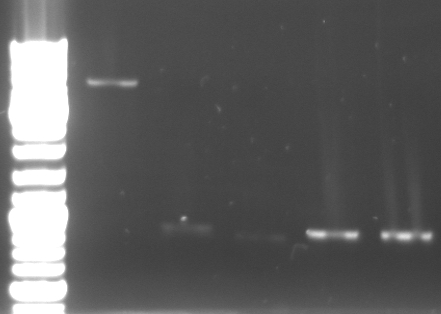
- lane0: DNA ladder mix
- lane1: purified 5.1kb band from last gel (pBluescript backbone from phage cloning strategy one (KpnI/AgeI)
- lane2: CmR1 digested (KpnI/SacI)
- lane3: CmR2 digested (KpnI/SacI)
- lane4: GFP1 digested (SacI/AgeI)
- lane5: GFP2 digested (SacI/AgeI)
- ligation of purified 5.1kb band from last gel (pBluescript backbone from phage cloning strategy one (KpnI/AgeI) with CmR1/2 and GFP1/2
- 30min at room temperature
- transformation in TOP10
Wednesday, 10/22/08
Proceedings of phage cloning strategy one
- first small colonies can be seen on chloramphenicol plates.
- colonies were picked and transferred in liquid media containing chloramphenicol
Thuresday, 10/23/08
Proceedings of phage cloning strategy one
- colonies could grow in chloramphenicol containing medium --> samll aliquots were transferred in ampicillin containing medium to check for the resisitance
- colony PCRs were conducted to ensure that our insert is embedded in the growing cells
- pUB307 was transferred in cells harboring our modified lambda phage by conjugation --> plating on Cm + Kan
Friday, 10/24/08
Proceedings of phage cloning strategy one
- colonies grew on plates containing chloramphenicol and kanamycin --> were picked and liquid medium inoculated
- conjugation of our lambda phage into cells harboring T9002 in pSB1A3 --> brought out on plates containing chloramphenicol and ampicillin
Saturday, 10/25/08
Proceedings of phage cloning strategy one
- we got colonies on the plates with ampicillin and chloramphenicol only when our conjugated cultures were brought out, but not if T9002 or the cells harboring pUB307 and our phage were brought out separately
- this shows that our phage can be transported by conjugation
| << Week 11 | Overview | Week 13 >> |
|---|
 "
"