Team:Brown/Project/Strategy
From 2008.igem.org
(→Electrical Reporting) |
(→Electrical Reporting) |
||
| (2 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
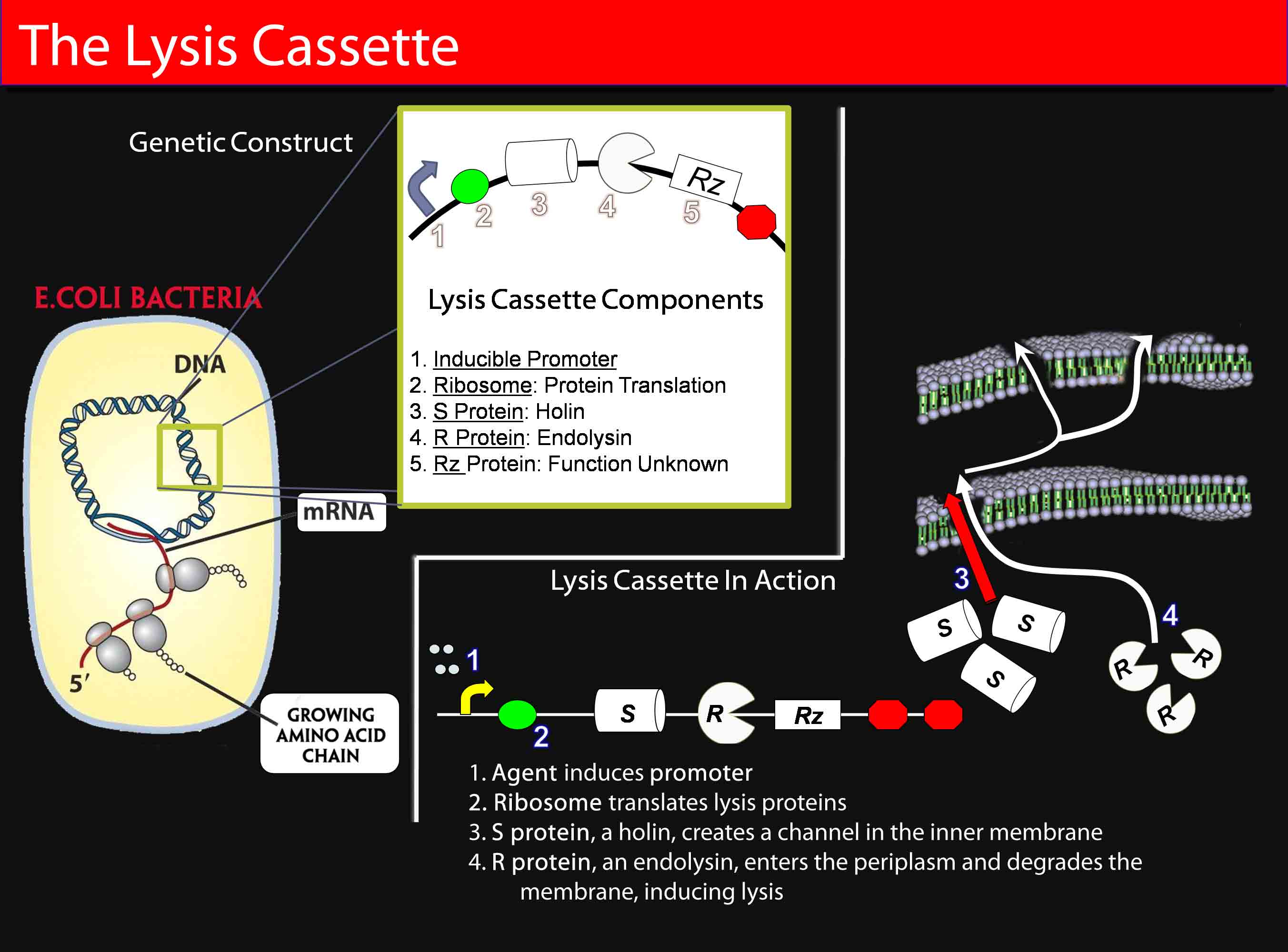
====Inducible Promoter==== | ====Inducible Promoter==== | ||
| - | + | [[Image:Arabinose20per.jpg|right|thumb|150px|We used Arabinose to induce the pBAD promoter on PVJ4.]] | |
In order to test the efficacy of the “lysis cassette,” our team first took advantage of the well-characterized pBAD promoter. We obtained the lysis cassette from the Mekalanos lab at Harvard Medical School on the pBAD18 plasmid. | In order to test the efficacy of the “lysis cassette,” our team first took advantage of the well-characterized pBAD promoter. We obtained the lysis cassette from the Mekalanos lab at Harvard Medical School on the pBAD18 plasmid. | ||
| Line 16: | Line 16: | ||
The lysis cassette consists of the S,R, and Rz genes under the control of a single promoter. These genes occur naturally in the lytic bacteriophage in an overlapping cluster downstream of the promoter P’r and lead to a specifically timed lytic event in the phage’s infective cycle. In bacteriophages, both the S105 and the S107 proteins are created, the S107 protein being an “anti-holin” that disrupts the activity of the S105 protein, the “lethal lysis effector.” The S107 gene has been deleted from the gene cassette used in experimentation. | The lysis cassette consists of the S,R, and Rz genes under the control of a single promoter. These genes occur naturally in the lytic bacteriophage in an overlapping cluster downstream of the promoter P’r and lead to a specifically timed lytic event in the phage’s infective cycle. In bacteriophages, both the S105 and the S107 proteins are created, the S107 protein being an “anti-holin” that disrupts the activity of the S105 protein, the “lethal lysis effector.” The S107 gene has been deleted from the gene cassette used in experimentation. | ||
| - | The S protein ('''S105 gene''') is a holin protein that inserts an unspecific inner membrane channel (M[r]= 8,500) in the cell membrane of the bacteria. The S proteins accumulate within the cell and cause membrane lesions simultaneously some time after translation. The lesion halts respiration, enables small molecules to leave the cell, and causes the cell to die (not lyse), even in the absence of an endolysin protein. | + | '''The S protein''' ('''S105 gene''') is a holin protein that inserts an unspecific inner membrane channel (M[r]= 8,500) in the cell membrane of the bacteria. The S proteins accumulate within the cell and cause membrane lesions simultaneously some time after translation. The lesion halts respiration, enables small molecules to leave the cell, and causes the cell to die (not lyse), even in the absence of an endolysin protein. |
The '''R gene''' (endolysin)encodes for a soluble transglycosylase. The S105 channels permeabilize the cell membrane to these proteins, allowing R proteins access to the periplasm. The protein attacks the peptidoglycan of the cell wall, inducing lysis. | The '''R gene''' (endolysin)encodes for a soluble transglycosylase. The S105 channels permeabilize the cell membrane to these proteins, allowing R proteins access to the periplasm. The protein attacks the peptidoglycan of the cell wall, inducing lysis. | ||
| Line 24: | Line 24: | ||
==Electrical Reporting== | ==Electrical Reporting== | ||
| - | When the bacterial cells lyse, the intracellular bulk ionic content of the cells is expelled to the extra-cellular solution, thereby | + | When the bacterial cells lyse, the intracellular bulk ionic content of the cells is expelled to the extra-cellular solution, thereby changing the current of the solution. Our team used both resistance and conductance measurements to measure this change. The method of current measurement is explained in greater detail in the "Testing" page. |
Latest revision as of 15:38, 29 October 2008
Components of Auto-Lysis DeviceOverviewInducible PromoterIn order to test the efficacy of the “lysis cassette,” our team first took advantage of the well-characterized pBAD promoter. We obtained the lysis cassette from the Mekalanos lab at Harvard Medical School on the pBAD18 plasmid. In the future, the team would like to test the ability of lead, mercury, arsenic, and xylene inducible promoters to induce the lysis cassette in the presence of the respective toxins. Lysis CassetteThe lysis cassette consists of the S,R, and Rz genes under the control of a single promoter. These genes occur naturally in the lytic bacteriophage in an overlapping cluster downstream of the promoter P’r and lead to a specifically timed lytic event in the phage’s infective cycle. In bacteriophages, both the S105 and the S107 proteins are created, the S107 protein being an “anti-holin” that disrupts the activity of the S105 protein, the “lethal lysis effector.” The S107 gene has been deleted from the gene cassette used in experimentation. The S protein (S105 gene) is a holin protein that inserts an unspecific inner membrane channel (M[r]= 8,500) in the cell membrane of the bacteria. The S proteins accumulate within the cell and cause membrane lesions simultaneously some time after translation. The lesion halts respiration, enables small molecules to leave the cell, and causes the cell to die (not lyse), even in the absence of an endolysin protein. The R gene (endolysin)encodes for a soluble transglycosylase. The S105 channels permeabilize the cell membrane to these proteins, allowing R proteins access to the periplasm. The protein attacks the peptidoglycan of the cell wall, inducing lysis. The Rz gene encodes for a gene of unknown function and is not necessary for lysis in laboratory conditions unless the cell membrane is stabilized by high concentrations of divalent ions. (1) Electrical ReportingWhen the bacterial cells lyse, the intracellular bulk ionic content of the cells is expelled to the extra-cellular solution, thereby changing the current of the solution. Our team used both resistance and conductance measurements to measure this change. The method of current measurement is explained in greater detail in the "Testing" page. |
 "
"