Team:Valencia/Project
From 2008.igem.org
| (34 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | <html>< | + | <html><link rel="stylesheet" href="https://2008.igem.org/wiki/index.php?title=User:Joadelas/valencia.css&action=raw&ctype=text/css" type="text/css"></html> |
| - | + | {{Valencia/Menu}} | |
| - | + | {{Valencia/Menu_Project}} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | & | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | <div class="valenciaMain"> | ||
<div style=" width:94%; margin: 0 auto;"> | <div style=" width:94%; margin: 0 auto;"> | ||
| Line 17: | Line 9: | ||
Heat is vital for life. Many living forms are unable to maintain its temperature in an adequate range; while others keep it constant using several biochemical mechanisms. We thought it would be really interesting to implement some of these biological tools in an organism that we could easily control. | Heat is vital for life. Many living forms are unable to maintain its temperature in an adequate range; while others keep it constant using several biochemical mechanisms. We thought it would be really interesting to implement some of these biological tools in an organism that we could easily control. | ||
| - | Using yeast as our model, we aim to be able to express a common mammal protein, | + | Using yeast as our model, we aim to be able to express a common mammal protein, thermogenin, to produce heat. This process would be really useful in many industrial applications and even daily routine actions. Thermogenin is a mitochondrial membrane protein that dissipates proton gradient in heat. |
We are using ''Saccharomyces cerevisiae'' strains kindly handed over by [http://www.cib.csic.es/en/grupo.php?idgrupo=39 Dr. Eduardo Rial]. Besides, we have built our own calorimeters so as to record temperature differences. | We are using ''Saccharomyces cerevisiae'' strains kindly handed over by [http://www.cib.csic.es/en/grupo.php?idgrupo=39 Dr. Eduardo Rial]. Besides, we have built our own calorimeters so as to record temperature differences. | ||
| - | In a later stage, we expect to control these temperature differences to optimize the possible applications. Consequently, we will implement a regulatory system for the | + | In a later stage, we expect to control these temperature differences to optimize the possible applications. Consequently, we will implement a regulatory system for the thermogenin gene expression. |
| + | Watch out for news from our project! | ||
| + | |||
| + | == How does thermogenin work? == | ||
<br> | <br> | ||
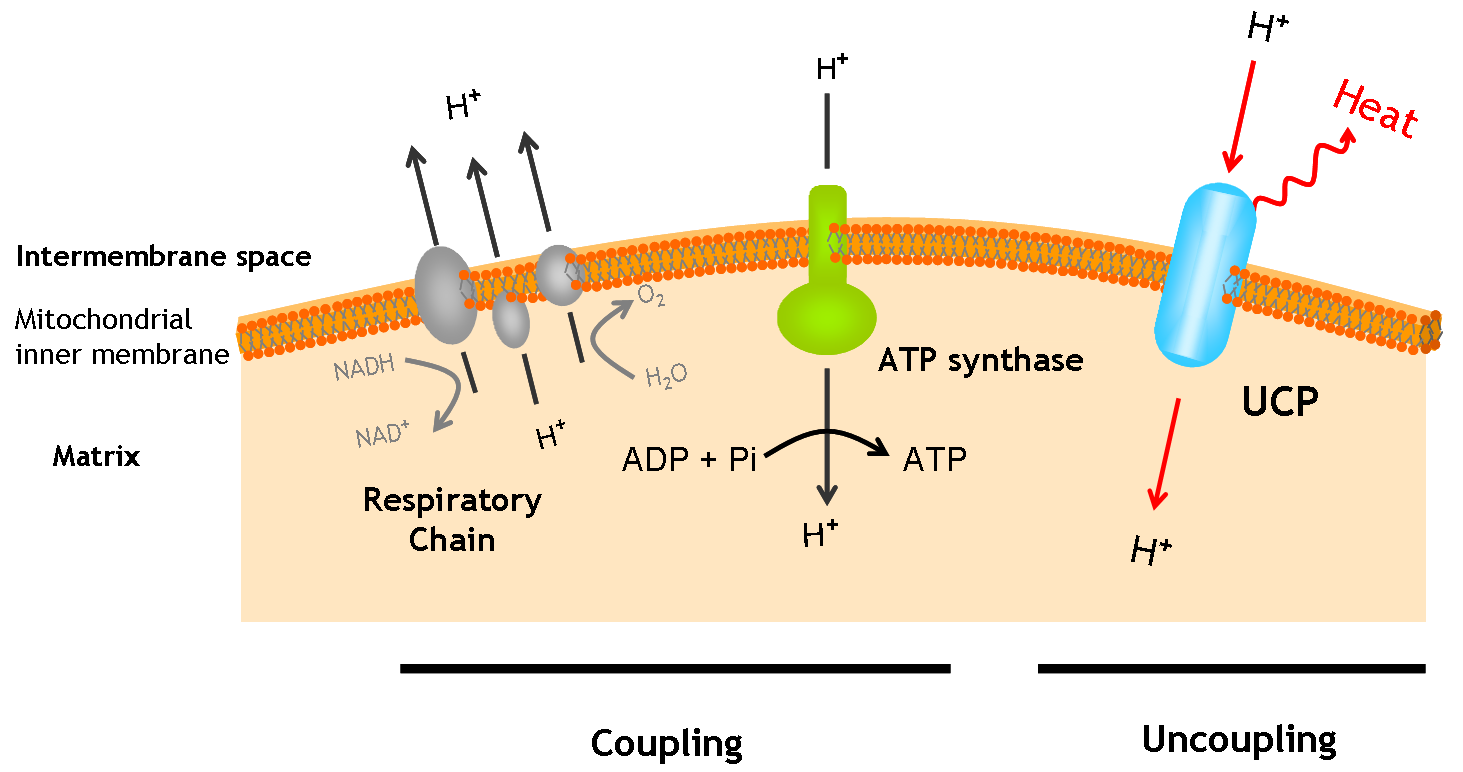
| + | UCP1, also known as thermogenin, is an uncoupling protein found in the mitochondria of brown adipose tissue. This protein plays an important role in hibernating mammals and in human infants since it is able to generate heat. UCP1 provides an alternative pathway by which protons can reenter the mitochondrial matrix from the intermembrane space, instead of going through ATP synthase. The result is a temperature increase rather than ATP production in the tissue containing this protein family. | ||
| + | |||
| + | [http://www.cib.csic.es/en/grupo.php?idgrupo=39 More information] | ||
| + | |||
| + | [[Image:Valencia_UCP.png|600px|center| UCP function in mitochondria]] | ||
| + | |||
| + | |||
| + | |||
| + | == Applications == | ||
| + | |||
| + | Maybe some of you are wondering: | ||
| + | |||
| + | What a yeast strain able to control its internal temperature could be useful for? | ||
| + | |||
| + | First, we just thought this idea would be interesting as a theoretical project. Nevertheless, when our group was running a brainstorming to see how to carry out the project and which could be the possible fields of application for this kind of processes, we found out that: | ||
| + | |||
| + | * One could have a living cell which maintains its temperature between certain levels without the need of introducing external heat to the system. Considering that enzymatic activity usually has a strong dependence on the temperature, this system would allow the reduction of electricity costs in an specific reaction. | ||
| + | |||
| + | * One could combine a heat-producing system such as the hot yeast strains we report here with other microbial cultures in order to heat them up. Since nutrients are converted into heat through uncoupling proteins, electricity-based heat production is saved. | ||
| + | |||
| + | * One could even implement this system in some plants species. If a plant could control its temperature it would be able to grow in colder climates or to survive frosts. | ||
| + | |||
| + | |||
| + | == References == | ||
| + | |||
| + | |||
| + | -Bandell M., Macpherson L. J. and Patapoutian A. (2007). From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. ''Current opinion in neurobiology'', '''17''', 490-497. | ||
| + | |||
| + | |||
| + | -Chomiki N., Voss J. C. and Warden C. H. (2001). EPR analysis and retinoic acid activation of UCP2. ''European Journal of Biochemistry '', '''268''' 903-913. | ||
| + | |||
| + | |||
| + | -Gonzalez-Barroso M. M., Fleury C., Rial E. et al. (1996). Activation of the Uncoupling Protein by Fatty Acids is Modulated by Mutations in the C-Terminal Region of the Protein. ''European Journal of Biochemistry'' '''239''' 445-450. | ||
| + | |||
| + | |||
| + | -González-Barroso M. M., Fleury C., Rial E. et al. (1999). Structural and functional study of a conserved region in the uncoupling protein UCP1: the three matrix loops are involved in the control of transport. ''Journal of Molecular Biology'' '''292''' 137-149. | ||
| + | |||
| + | |||
| + | -Hinz W., Faller B., Grüninger S. et al. (1999). Recombinant human uncoupling protein-3 increases thermogenesis in yeast cells. ''FEBS letters'' '''448''' 57-61. | ||
| + | |||
| + | |||
| + | -Ledesma A., Lacoba M. G. , Rial E. et al. (2002). Modeling the Transmembrane Arrangement of the Uncoupling Protein UCP1 and Topological Considerations of the Nucleotide-Binding Site. ''Journal of Bioenergetics and Biomembranes'' '''34''' 473-486. | ||
| + | |||
| + | |||
| + | -Ledesma A., de Lacoba M. G. and Rial E. (2002). The mitochondrial uncoupling proteins. ''Genome biology'' '''3''' Review3015. | ||
| + | |||
| + | |||
| + | -Tomás P., Jiménez-Jiménez J., Rial E. et al. (2004). Activation by retinoids of the uncoupling protein UCP1. ''BBA-Bioenergetics'' '''1658''' 157-164. | ||
| + | |||
| + | |||
| + | - Gonçalves, T. and Lourero-Dias M., (1994) Aspects of glucose uptake in ''Saccharomyces cerevisiae''. ''American society for microbiology'' '''176''' 1511-1513. | ||
<br> | <br> | ||
| + | <div style="padding: 10px; width: 200px; color: #000000; background-color: #FFB428"> | ||
| + | <center><font face="trebuchet ms" style="color:#047DB5" size="3">'''Introduction '''>> [[Team:Valencia/Project/Objectives | <font color="#047DB5">'''Objectives'''</font>]]</font> | ||
| + | </center> | ||
| + | </div style> | ||
| + | |||
| + | |||
| + | </div> | ||
</div> | </div> | ||
Latest revision as of 19:16, 29 October 2008
Heat is vital for life. Many living forms are unable to maintain its temperature in an adequate range; while others keep it constant using several biochemical mechanisms. We thought it would be really interesting to implement some of these biological tools in an organism that we could easily control.
Using yeast as our model, we aim to be able to express a common mammal protein, thermogenin, to produce heat. This process would be really useful in many industrial applications and even daily routine actions. Thermogenin is a mitochondrial membrane protein that dissipates proton gradient in heat.
We are using Saccharomyces cerevisiae strains kindly handed over by [http://www.cib.csic.es/en/grupo.php?idgrupo=39 Dr. Eduardo Rial]. Besides, we have built our own calorimeters so as to record temperature differences.
In a later stage, we expect to control these temperature differences to optimize the possible applications. Consequently, we will implement a regulatory system for the thermogenin gene expression.
Watch out for news from our project!
How does thermogenin work?
UCP1, also known as thermogenin, is an uncoupling protein found in the mitochondria of brown adipose tissue. This protein plays an important role in hibernating mammals and in human infants since it is able to generate heat. UCP1 provides an alternative pathway by which protons can reenter the mitochondrial matrix from the intermembrane space, instead of going through ATP synthase. The result is a temperature increase rather than ATP production in the tissue containing this protein family.
[http://www.cib.csic.es/en/grupo.php?idgrupo=39 More information]
Applications
Maybe some of you are wondering:
What a yeast strain able to control its internal temperature could be useful for?
First, we just thought this idea would be interesting as a theoretical project. Nevertheless, when our group was running a brainstorming to see how to carry out the project and which could be the possible fields of application for this kind of processes, we found out that:
- One could have a living cell which maintains its temperature between certain levels without the need of introducing external heat to the system. Considering that enzymatic activity usually has a strong dependence on the temperature, this system would allow the reduction of electricity costs in an specific reaction.
- One could combine a heat-producing system such as the hot yeast strains we report here with other microbial cultures in order to heat them up. Since nutrients are converted into heat through uncoupling proteins, electricity-based heat production is saved.
- One could even implement this system in some plants species. If a plant could control its temperature it would be able to grow in colder climates or to survive frosts.
References
-Bandell M., Macpherson L. J. and Patapoutian A. (2007). From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Current opinion in neurobiology, 17, 490-497.
-Chomiki N., Voss J. C. and Warden C. H. (2001). EPR analysis and retinoic acid activation of UCP2. European Journal of Biochemistry , 268 903-913.
-Gonzalez-Barroso M. M., Fleury C., Rial E. et al. (1996). Activation of the Uncoupling Protein by Fatty Acids is Modulated by Mutations in the C-Terminal Region of the Protein. European Journal of Biochemistry 239 445-450.
-González-Barroso M. M., Fleury C., Rial E. et al. (1999). Structural and functional study of a conserved region in the uncoupling protein UCP1: the three matrix loops are involved in the control of transport. Journal of Molecular Biology 292 137-149.
-Hinz W., Faller B., Grüninger S. et al. (1999). Recombinant human uncoupling protein-3 increases thermogenesis in yeast cells. FEBS letters 448 57-61.
-Ledesma A., Lacoba M. G. , Rial E. et al. (2002). Modeling the Transmembrane Arrangement of the Uncoupling Protein UCP1 and Topological Considerations of the Nucleotide-Binding Site. Journal of Bioenergetics and Biomembranes 34 473-486.
-Ledesma A., de Lacoba M. G. and Rial E. (2002). The mitochondrial uncoupling proteins. Genome biology 3 Review3015.
-Tomás P., Jiménez-Jiménez J., Rial E. et al. (2004). Activation by retinoids of the uncoupling protein UCP1. BBA-Bioenergetics 1658 157-164.
- Gonçalves, T. and Lourero-Dias M., (1994) Aspects of glucose uptake in Saccharomyces cerevisiae. American society for microbiology 176 1511-1513.
 "
"