Team:ETH Zurich/Modeling/Framework
From 2008.igem.org
(→Summary of the Algorithm and Interplay of Frameworks Componets) |
(→Detailed description of the Modeling Framework) |
||
| Line 26: | Line 26: | ||
#:We model a chemostat using a system of coupled differential equations for a population of different mutants with different growth rates competing for a limited external thymidine concentration. By varying the time intervals between two pulses of restriction enzymes (where only a small amount of previous population survived and new mutants are generated) we control the number of different mutants considering for further gene deletions after the next pulse event, consequently the diversity of a population. | #:We model a chemostat using a system of coupled differential equations for a population of different mutants with different growth rates competing for a limited external thymidine concentration. By varying the time intervals between two pulses of restriction enzymes (where only a small amount of previous population survived and new mutants are generated) we control the number of different mutants considering for further gene deletions after the next pulse event, consequently the diversity of a population. | ||
# '''Switch generator for short-time Restriction Enzyme Expression''' | # '''Switch generator for short-time Restriction Enzyme Expression''' | ||
| - | #:Using a novel pulsing mechanism consisting of two signals – a start signal that initiates the synthesis of restriction enzymes, and a stop signal, which switches gene expression off - we are able to delete genome fragments in vivo. To simulate this system we developed a switch circuit which works | + | #:Using a novel pulsing mechanism consisting of two signals – a start signal that initiates the synthesis of restriction enzymes, and a stop signal, which switches gene expression off - we are able to delete genome fragments in vivo. To simulate this system we developed a [[Team:ETH_Zurich/Modeling/Switch_Circuit#Switch_Circuit switch circuit]] which works as follows: Restriction enzyme gene expression is under the control of LacI and can be induced by the addition of IPTG. In order to stop gene expression, the IPTG-sensitive LacI is replaced by IPTG-insensitive LacIS, which shuts restriction enzyme gene expression off again. The synthesis of LacIS is started by the addition of Tetracyclin to the system, which binds to the tet repressor TetR and thus de-represses the expression of the lacIS gene. This circuit is modeled by ca. 40 reactions and is simulated using ODE solver and stochastic simulations |
===== Summary of the Algorithm and Interplay of Frameworks Componets ===== | ===== Summary of the Algorithm and Interplay of Frameworks Componets ===== | ||
Revision as of 22:29, 29 October 2008
|
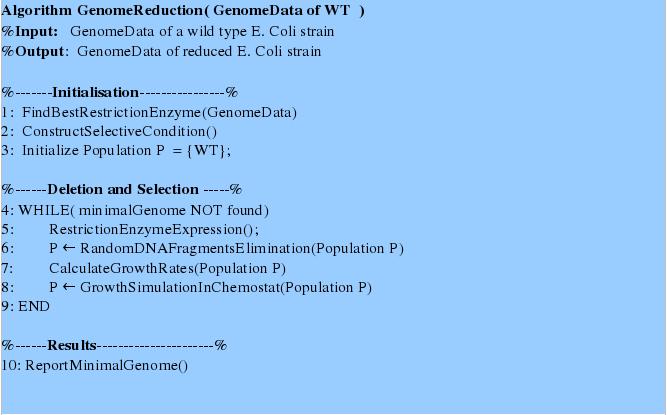
We propose a novel method of random gene deletion and chemostat-based selection of species with a reduced genome. For this we provide an algorithm described below. Modeling FrameworkThis algorithm requires a modeling framework consisting of four main parts:
Detailed description of the Modeling Framework
Summary of the Algorithm and Interplay of Frameworks ComponetsFirst tree steps in the algorithm initialize and prepare the system for gene deletions and growth simulations. In the first step the genome data are analyzed in order to find a most suitable restriction enzyme for random fragments deletion using the “Restriction Enzyme Analysis”- procedure. For the second step the state-of-the art model is adjusted by introducing a selective pressure due to the genome size. Thirdly, initial population is set to consist of one type, namely wildtype. The next steps of the algorithm perform in depth genome fragments deletion and growth simulation. First, we simulate the restriction enzyme expression and the consecutive population decline and occurrence of new mutants with reduced genome. The growth rates of the latter can be predicted using Flux Balance Analysis. For these simulations we used the framework parts: “Switch generator” and “Flux Balance Analysis on a Genome Scale Model “. Secondly, the growth simulations are performed using a chemostat model and the distribution of different mutant types according to the growth rates obtained. This simulation continues until no better mutants is be generated. Eventually, the genome data of the fastest growing reduced genome mutant can be returned. |
 "
"