Team:Hawaii/Notebook/2008-07-10
From 2008.igem.org
(Difference between revisions)
(→Drylab Work) |
(→Quote of the Day) |
||
| (2 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
= Things we did today = | = Things we did today = | ||
== Wetlab work == | == Wetlab work == | ||
| - | === | + | ===Growing up ''E. coli''=== |
| - | :<strong> | + | :<strong> Krystle</strong> |
| - | :* | + | :* Grew up single colonies of Biobrick harboring ''E. coli'' for cryostocking and plasmid prep tomorrow. |
| - | + | ||
| + | ===PCR=== | ||
| + | :<strong> Grace and Krystle</strong> | ||
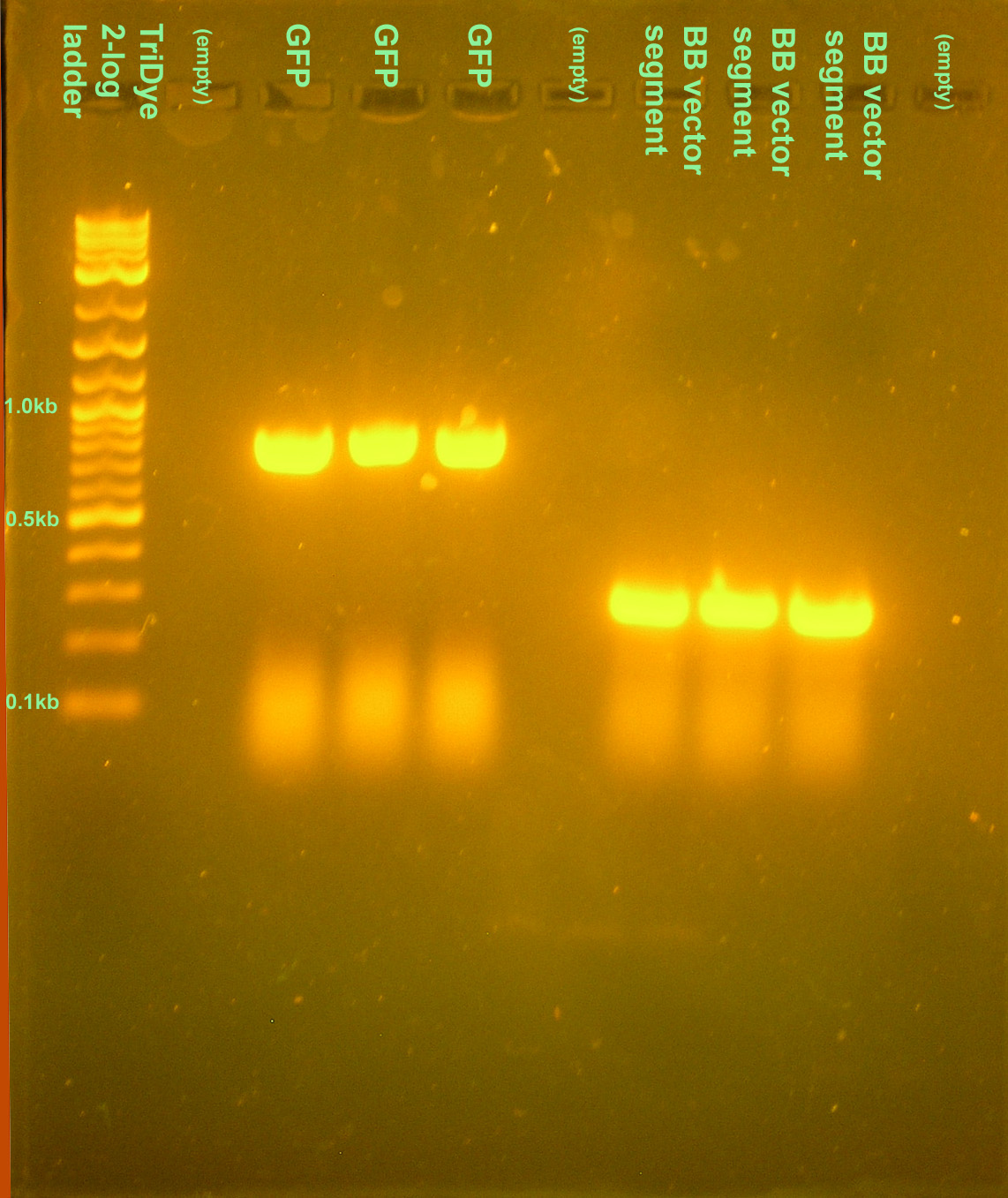
| + | [[Image:071108.jpg|right|thumb|150px|PCR products as verified on an EtBr stained 2% agarose gel ran at 95V for 30 min. Ten microliters of the PCR reaction were loaded into each well. Only one PCR product was visible in each lane corresponding the size of the desired product. Products will be confirmed by sequencing later.]] | ||
| + | :* Ran 10 μl PCR reactions with Green Taq for GFP site directed mutagenesis and priming out the Biobrick sites from BBa_C0012 (for construction of our own Biobrick vector) | ||
| + | ::* Forgot to keep everything on ice. May have non-specific amplifications. Will run on a gel tomorrow to check. | ||
| + | ===Redid ligation/restriction digest of annealed products=== | ||
| + | :<strong> Grace</strong> | ||
| + | |||
| + | :* Ligated 1:1 and 1:10 dilutions of annealed product (20-30 min ligation) then restriction digested w/ XbaI and PstI using full RE buffer amounts recommended by NEB (2.5 hours digest). | ||
| + | :* Heated digested products in 95C water bath for 10 min before running on gel. | ||
| + | :* Ran 3% gel with RE products (both ligation dilutions) and annealed but not ligated products. Gel still had ladders. See [[Team:Hawaii/Initial_Synth._Oligo_Assembly|experiment]]. | ||
| + | :* Ran 0.8% gel with 20 μl RE digested BBa_C0012 products. | ||
| + | :* Excised bands from gels for DNA purification/extraction tomorrow. | ||
= Discussion = | = Discussion = | ||
= Quote of the Day = | = Quote of the Day = | ||
| - | [[Image:20080710-combless gel electrophoresis.jpg|thumb| | + | [[Image:20080710-combless gel electrophoresis.jpg|thumb|left|we found this poured in the gel room today]] |
<blockquote>''Looks like someone is developing a comb-less gel electrophoresis protocol.'' - NW, KLS</blockquote> | <blockquote>''Looks like someone is developing a comb-less gel electrophoresis protocol.'' - NW, KLS</blockquote> | ||
Latest revision as of 09:10, 14 July 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Growing up E. coli
- Krystle
- Grew up single colonies of Biobrick harboring E. coli for cryostocking and plasmid prep tomorrow.
PCR
- Grace and Krystle
- Ran 10 μl PCR reactions with Green Taq for GFP site directed mutagenesis and priming out the Biobrick sites from BBa_C0012 (for construction of our own Biobrick vector)
- Forgot to keep everything on ice. May have non-specific amplifications. Will run on a gel tomorrow to check.
Redid ligation/restriction digest of annealed products
- Grace
- Ligated 1:1 and 1:10 dilutions of annealed product (20-30 min ligation) then restriction digested w/ XbaI and PstI using full RE buffer amounts recommended by NEB (2.5 hours digest).
- Heated digested products in 95C water bath for 10 min before running on gel.
- Ran 3% gel with RE products (both ligation dilutions) and annealed but not ligated products. Gel still had ladders. See experiment.
- Ran 0.8% gel with 20 μl RE digested BBa_C0012 products.
- Excised bands from gels for DNA purification/extraction tomorrow.
Discussion
Quote of the Day
Looks like someone is developing a comb-less gel electrophoresis protocol. - NW, KLS
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"