Team:Hawaii/Notebook/2008-07-30
From 2008.igem.org
(Difference between revisions)
(→Discussion) |
m (→Colony PCR) |
||
| (20 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
= Things we did today = | = Things we did today = | ||
== Wetlab work == | == Wetlab work == | ||
| + | |||
| + | === Restriction Digest=== | ||
| + | :<strong>Grace</strong> | ||
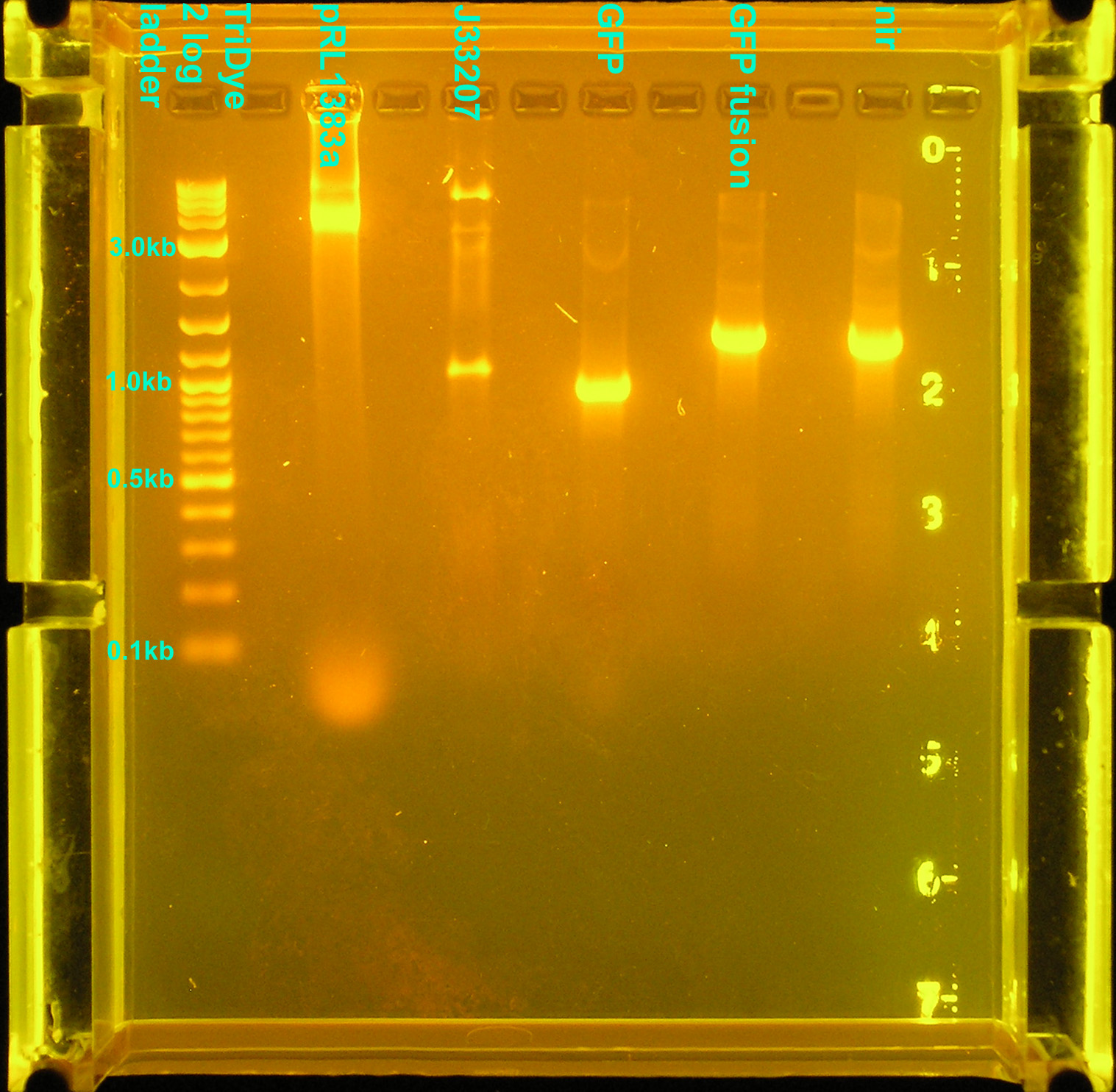
| + | [[Image:073008REdigests.jpg|thumb|right|200px|EtBr stained 1.2% agarose gel. Twenty microliters of the restriction digest reactions were loaded into each well. Gel ran at 59V for 1.5 hours.]] | ||
| + | :* Digested 5 μl GFP, GFPf, and nir PCR products with EcoRI and SpeI | ||
| + | :* Sequentially digested 10 μl pRL1383a and 5 μl J33207 PCR product with BamHI and HindIII | ||
| + | ::* Used Roche enzymes and Roche buffer M (buffer B also works) from SC | ||
| + | :* Ran digests on gel | ||
| + | ::* pRL1383a and J33207 bands good (excised) | ||
| + | ::* GFP, GFPf, and nir bands still too big | ||
| + | |||
| + | :<strong>Krystle</strong> | ||
| + | :* Digested 10 ul each of GFP, GFPf, and nir PCR products from Grace's PCR on 07/30/08 with EcoRI and SpeI | ||
| + | |||
| + | ===PCR=== | ||
| + | :<strong>Grace and Krystle</strong> | ||
| + | |||
| + | :* Redid yesterday's PCR of GFP, GFPf, and nir | ||
| + | :* Reran on gel, producing almost identical results. Bands were excised. | ||
| + | |||
===Gel Purification=== | ===Gel Purification=== | ||
:<strong> Grace</strong> | :<strong> Grace</strong> | ||
:* Purified B0030 and B0024 from gel | :* Purified B0030 and B0024 from gel | ||
| + | ::* B0030 = 21.9 ng/μl | ||
| + | ::* B0024 = 5.7 ng/μl | ||
| - | + | :<strong> Krystle</strong> | |
| - | :<strong> | + | :* Purified restricted pRL1383 and J33207 from gel |
| + | :* Purified Grace's PCR products of GFP, GFPfusion, and nir from gel | ||
| + | ::* Why are bands still ~1kb and ~1.2kb? | ||
| - | : | + | ===Plasmid Prep=== |
| - | :* | + | :<strong> Krystle</strong> |
| - | :: | + | :* Maxiprep of pRL1383a |
| - | :* | + | |
| + | ===[[Team:Hawaii/PCR Amplification of pRL1383a|PCR amplification of pRL1383a]]=== | ||
| + | :<strong> Margaret </strong> | ||
| + | [[Image:gel_PCR_7_30_08_oriV_aada_rep.jpg|right|thumb|150px|PCR amplification of oriV, aada, rep.]] | ||
| + | |||
| + | :* ran a gel from [[Team:Hawaii/Notebook/2008-07-29|yesterday's PCR]] amplification of oriV, aadA, and rep regions. | ||
| - | == | + | ===Mini-Prep=== |
| - | + | :<strong> Margaret </strong> | |
| - | :<strong> | + | |
| - | :* | + | :*Plasmid Prep of [http://partsregistry.org/Part:BBa_I51020 BBa_I51020],[http://partsregistry.org/Part:pSB1A7 pSB1A7], [http://partsregistry.org/Part:BBa_B0015 BBa_B0015] |
| - | : | + | |
| + | ===Colony PCR=== | ||
| + | :<strong> Margaret </strong> | ||
| + | [[Image:colony_PCR_I14032_lac_oriT.jpg|right|thumb|300px|A colony PCR verification of [http://partsregistry.org/Part:BBa_I14032 BBa_I14032], a lac Promoter and the oriT construct.]] | ||
| + | :* verify the insert of the oriT construct and [http://partsregistry.org/Part:BBa_I14032 BBa_I14032] | ||
| + | :* Results: | ||
| + | ::*[http://partsregistry.org/Part:BBa_I14032 BBa_I14032] should be 334bp, the band under 0.4kb indicating a verification of this size of insert. | ||
| + | ::*oriT should be about 415bp(+BioBrick ends), the band is around 0.4kb so is in approximately the right place. | ||
| + | :*Discussion: The a plasmid prep and glycerol stock of both will be made. I need to verify the oriT part through sequencing. | ||
= Discussion = | = Discussion = | ||
Latest revision as of 02:52, 2 August 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Restriction Digest
- Grace
- Digested 5 μl GFP, GFPf, and nir PCR products with EcoRI and SpeI
- Sequentially digested 10 μl pRL1383a and 5 μl J33207 PCR product with BamHI and HindIII
- Used Roche enzymes and Roche buffer M (buffer B also works) from SC
- Ran digests on gel
- pRL1383a and J33207 bands good (excised)
- GFP, GFPf, and nir bands still too big
- Krystle
- Digested 10 ul each of GFP, GFPf, and nir PCR products from Grace's PCR on 07/30/08 with EcoRI and SpeI
PCR
- Grace and Krystle
- Redid yesterday's PCR of GFP, GFPf, and nir
- Reran on gel, producing almost identical results. Bands were excised.
Gel Purification
- Grace
- Purified B0030 and B0024 from gel
- B0030 = 21.9 ng/μl
- B0024 = 5.7 ng/μl
- Krystle
- Purified restricted pRL1383 and J33207 from gel
- Purified Grace's PCR products of GFP, GFPfusion, and nir from gel
- Why are bands still ~1kb and ~1.2kb?
Plasmid Prep
- Krystle
- Maxiprep of pRL1383a
PCR amplification of pRL1383a
- Margaret
- ran a gel from yesterday's PCR amplification of oriV, aadA, and rep regions.
Mini-Prep
- Margaret
- Plasmid Prep of [http://partsregistry.org/Part:BBa_I51020 BBa_I51020],[http://partsregistry.org/Part:pSB1A7 pSB1A7], [http://partsregistry.org/Part:BBa_B0015 BBa_B0015]
Colony PCR
- Margaret
- verify the insert of the oriT construct and [http://partsregistry.org/Part:BBa_I14032 BBa_I14032]
- Results:
- [http://partsregistry.org/Part:BBa_I14032 BBa_I14032] should be 334bp, the band under 0.4kb indicating a verification of this size of insert.
- oriT should be about 415bp(+BioBrick ends), the band is around 0.4kb so is in approximately the right place.
- Discussion: The a plasmid prep and glycerol stock of both will be made. I need to verify the oriT part through sequencing.
Discussion
The gel purification kit is almost out of PE. If we need more before our order comes in, there is PE in the Qiaprep Spin Miniprep kit (just remember to add EtOH before use).
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"