Team:Hawaii/Notebook/2008-09- 4
From 2008.igem.org
(Difference between revisions)
(→Re-replacement of BB-pRL1383a MCS) |
(→Re-replacement of BB-pRL1383a MCS) |
||
| (3 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== Wetlab work == | == Wetlab work == | ||
===Re-replacement of BB-pRL1383a MCS=== | ===Re-replacement of BB-pRL1383a MCS=== | ||
| + | |||
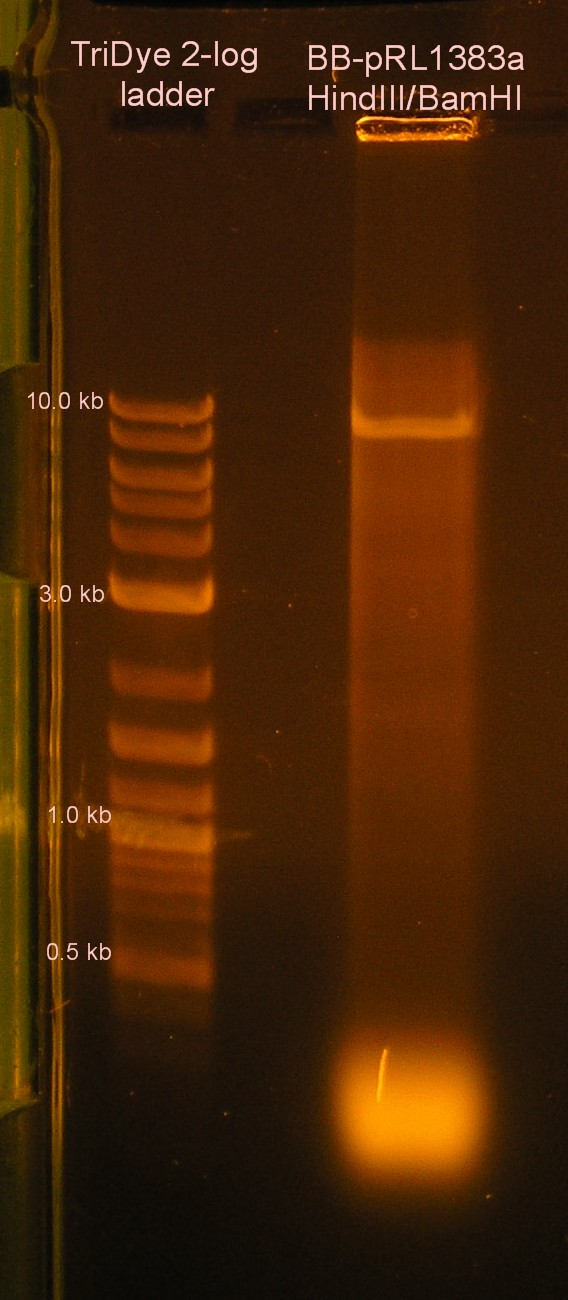
| + | [[Image:090408BBpRL.jpg|right|thumb|140px|EtBr stained 0.8% agarose gel ran at 60V for 1.5 hours. Thirty microliters of RE digested BB-pRL1383a were loaded onto the gel.]] | ||
:<strong> Grace</strong> | :<strong> Grace</strong> | ||
| - | |||
:* Ran last night's RE digest on agarose gel | :* Ran last night's RE digest on agarose gel | ||
:* Extracted J33207 and BB-pRL1383a from gel | :* Extracted J33207 and BB-pRL1383a from gel | ||
| Line 23: | Line 24: | ||
:* Transformed into DB3.1 cells | :* Transformed into DB3.1 cells | ||
| - | == | + | === Cont'd construction of GFP + TT === |
| + | :<strong>Krystle</strong> | ||
| + | :* Transformed negative control and ligation product from Tuesday into DB3.1 | ||
| + | |||
| + | === Construction of B-H-R Vector Intermediates === | ||
| + | :<strong>Margaret </strong> | ||
| + | [[Image:re_gel_9_3.jpg|right|thumb|300px|Re-digest of RBS+Promoters, aada, psb3k3, psb1a3, J33207, and oriT's.]] | ||
| + | :*Ran a gel of [[Team:Hawaii/Notebook/2008-09- 3|Yesterday's]] restriction digest | ||
| + | :*Purified the bands that were the correct size, will re-do the others. | ||
| + | :*Set up cultures of constructs that did not appear on the gel. | ||
| - | |||
| - | |||
| - | |||
| - | |||
Latest revision as of 01:42, 6 September 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Things we did today
Wetlab work
Re-replacement of BB-pRL1383a MCS
- Grace
- Ran last night's RE digest on agarose gel
- Extracted J33207 and BB-pRL1383a from gel
- Treated BB-pRL1383a w/ SAP
- Checked DNA concentration of BB-pRL1383a (---ng/μl)
- Ligated J33207 with BB-pRL1383a
- Negative controls = BB-pRL1383a ligated to self, J33207 ligated to self
- Transformed into DH5α cells
3A construction of plac+rbs
- Grace
- Ligated:
- C0012V (SAP treated) + plac + rbs (B0034 back ligation)
- C0012V + plac + rbs (B0034 back ligation)
- Negative controls = C0012V (SAP treated and no SAP treatment) ligated to self, plac + rbs ligated w/o vector
- Transformed into DB3.1 cells
Cont'd construction of GFP + TT
- Krystle
- Transformed negative control and ligation product from Tuesday into DB3.1
Construction of B-H-R Vector Intermediates
- Margaret
File:Re gel 9 3.jpg
Re-digest of RBS+Promoters, aada, psb3k3, psb1a3, J33207, and oriT's.
- Ran a gel of Yesterday's restriction digest
- Purified the bands that were the correct size, will re-do the others.
- Set up cultures of constructs that did not appear on the gel.
Discussion
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"