Team:Caltech/Protocols/Folate assay
From 2008.igem.org
(Difference between revisions)
(→L. rhamnosus inoculum preparation) |
|||
| (10 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | {{Caltech_iGEM_08| | |
| - | + | Content= | |
| - | + | ||
| - | {{Caltech_iGEM_08 | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==Folate Microbiological Assay | + | <div style="font-size:18pt;"> |
| + | <font face="verdana" style="color:#CC3300">Folate Microbiological Assay</font></div> | ||
| + | <br> | ||
| + | __TOC__ | ||
===Purpose=== | ===Purpose=== | ||
To quantify and measure levels of folate production in the cell lysate and supernatant of 'E.coli' transformed with high copy folate biosynthesis genes ''folB,'' ''folKE,'' ''folBKE''. | To quantify and measure levels of folate production in the cell lysate and supernatant of 'E.coli' transformed with high copy folate biosynthesis genes ''folB,'' ''folKE,'' ''folBKE''. | ||
| - | The assay uses growth of the folate-dependent strain ''L. rhamnosus'' as an indicator of folate | + | The assay uses growth of the folate-dependent strain ''L. rhamnosus'' as an indicator of folate concentration in the sample. Growth is measured by taking the OD at 546nm. |
===Important Notes=== | ===Important Notes=== | ||
| - | *Almost every ingredient in this assay is light sensitive- take extreme caution to limit exposure of buffers, folic acid mixtures, and sample tubes to light. I usually wrapped the buffers, assay media, and standard curve folic acid dilutions in aluminum foil. I also started covering the entire rack of samples with foil during incubation, but decided it would be ridiculous to covered each sample tube with foil. | + | *Almost every ingredient in this assay is light sensitive -- take extreme caution to limit exposure of buffers, folic acid mixtures, and sample tubes to light. I usually wrapped the buffers, assay media, and standard curve folic acid dilutions in aluminum foil. I also started covering the entire rack of samples with foil during incubation, but decided it would be ridiculous to covered each sample tube with foil. |
| - | * Previously, we had been using this protocol with the other folate-dependent strain ''Entercoccus hirae'' ATCC 8043, and were able to generate a linear standard curve from 0.1- | + | * Previously, we had been using this protocol with the other folate-dependent strain ''Entercoccus hirae'' ATCC 8043, and were able to generate a linear standard curve from 0.1-1 ng. However, this strain did not grow when combined with our samples, so we switched to ''L. rhamnosus''. Lactobacilli Broth AOAC is recommended for ''L. rhamnosus'', so we used this media in place of the recommended Lactobacilli MRS broth. |
| - | + | ||
| - | + | ||
===Materials=== | ===Materials=== | ||
* Folic Acid Assay Medium (BD Biosciences) | * Folic Acid Assay Medium (BD Biosciences) | ||
| - | * Lactobacilli Broth AOAC | + | * Lactobacilli Broth AOAC (BD Biosciences) |
* Folic Acid (for standard curve) | * Folic Acid (for standard curve) | ||
* 0.1M sodium acetate buffer (pH4.8) -1% ascorbic acid | * 0.1M sodium acetate buffer (pH4.8) -1% ascorbic acid | ||
| Line 53: | Line 47: | ||
===Sample Preparation=== | ===Sample Preparation=== | ||
| - | + | # Centrifuge the full-grown cell culture (5ml) after centrifugation (13,000x g, 10 min, 20 C). | |
| - | + | # Recover both cells and supernatant. | |
| - | + | # Dilute the supernatant 1:1 with 0.1M sodium acetate buffer (pH4.8) -1% ascorbic acid. | |
| - | + | # Wash with the 0.1M sodium acetate-1% ascorbic acid and resuspend in 5 mL of the same buffer. | |
| - | + | # Release folate from the cells by incubating the samples at 100C for 5 min (determined to be optimal for folate release + the heat inactivates the bacteria) | |
| - | + | # Add the deconjugation reaction mixture (2.5% vol/vol) | |
| - | + | # Incubate for 4h at 37C, you should see obvious cell debris at the bottom of the cell lysate tubes. | |
| - | + | # Filter sterilize 2.5 mL of each sample into a clean glass tube. | |
| - | + | # Add 2.5 mL of folate assay media to each tube, bringing the volume up to 5mL. | |
| - | + | # Add 50uL of prepared ''L.rhamnosus'' inoculum to each assay tube. | |
| - | + | # Incubate for 16-20 hours at 37C. | |
| - | + | # Read the absorbances of the samples at 546 nm on a plate reader (200uL per well). The protocol recommends refrigerating the samples at 4C for 15-30 minutes prior to reading, but I don't think it makes a difference, particularly if you intend on returning the samples to the incubator to take later timepoints. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
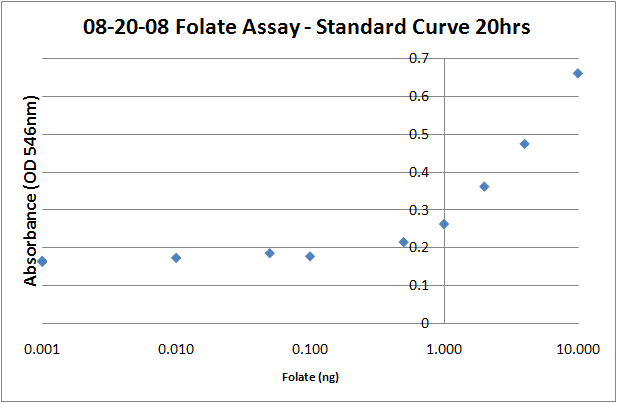
===Standard Curve=== | ===Standard Curve=== | ||
| - | + | # Prepare folic acid dilutions from 0- 10 ng/mL. Usually we used 0.001, 0.01, 0.05, 0.1, 0.5, 1, 2, 4, 8, 10ng samples. With ''L. rhamnosus'' the linear range of the assay appears to be from 0.1 to 10 ng. | |
| - | + | # Add 2.5 mL of folic acid assay media to each tube, along with 2.5 mL folic acid + water to make up a total volume of 5mL. | |
| - | + | # Add 50uL of the prepared ''L. rhamnosus'' inoculum. | |
| - | + | # Incubate for 16-20 hours at 37C. | |
| - | + | # Read the absorbances of the samples at 546 nm on a plate reader (200uL per well). The protocol recommends refrigerating the samples at 4C for 15-30 minutes prior to reading, but I don't think it makes a difference, particularly if you intend on returning the samples to the incubator to take later timepoints. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
[[Image:08-20-08_Folate_Assay_Std_20_hrs_log-lin.png|thumb|center| A fantastically linear standard curve]] | [[Image:08-20-08_Folate_Assay_Std_20_hrs_log-lin.png|thumb|center| A fantastically linear standard curve]] | ||
| Line 94: | Line 73: | ||
* Horne et al. ''Lactobacillus casei Microbiological Assay of Folic Acid Derivatives in 96-Well Microtiter Plates'' '''CLIN. CHEM.''' 34/11, 2357-2359 (1988) [http://www.clinchem.org/cgi/reprint/34/11/2357?ijkey=b6982c31167f9092795d87ca8041bf27c5fb7a09] | * Horne et al. ''Lactobacillus casei Microbiological Assay of Folic Acid Derivatives in 96-Well Microtiter Plates'' '''CLIN. CHEM.''' 34/11, 2357-2359 (1988) [http://www.clinchem.org/cgi/reprint/34/11/2357?ijkey=b6982c31167f9092795d87ca8041bf27c5fb7a09] | ||
* ''Folic Acid Assay Medium'' [http://www.bd.com/ds/technicalCenter/inserts/Folic_Acid_Assay_Medium.pdf] | * ''Folic Acid Assay Medium'' [http://www.bd.com/ds/technicalCenter/inserts/Folic_Acid_Assay_Medium.pdf] | ||
| - | + | ||
| + | ===Contacts=== | ||
| + | [[User:vhsiao|Victoria Hsiao]] | ||
| + | }} | ||
Latest revision as of 00:48, 30 October 2008
|
People
|
Folate Microbiological Assay
PurposeTo quantify and measure levels of folate production in the cell lysate and supernatant of 'E.coli' transformed with high copy folate biosynthesis genes folB, folKE, folBKE. The assay uses growth of the folate-dependent strain L. rhamnosus as an indicator of folate concentration in the sample. Growth is measured by taking the OD at 546nm. Important Notes
Materials
Folic Acid Assay Media
Deconjugation Mixture
L. rhamnosus inoculum preparation
Sample Preparation
Standard Curve
Sources
Contacts |
 "
"