Team:Hawaii/PCC6803 Electroporation of PCC6803
From 2008.igem.org
(Difference between revisions)
m (→Discussion) |
m |
||
| (One intermediate revision not shown) | |||
| Line 17: | Line 17: | ||
# determine number of viable cells after electroporation: 10^-4 dilution made before collecting the culture, 50 uL of this dilution plated with out selection, incubate 14 days, count colonies. Total viable cells (colonies grown on non-selective plates * 10^7) and total number transformants (colonies on selective plates) determined | # determine number of viable cells after electroporation: 10^-4 dilution made before collecting the culture, 50 uL of this dilution plated with out selection, incubate 14 days, count colonies. Total viable cells (colonies grown on non-selective plates * 10^7) and total number transformants (colonies on selective plates) determined | ||
| - | + | <strong> Cultivation </strong> | |
| - | + | *''Synechocystis'' is photo-autotrophically grown in BG11 at 30°C under white light (Chauvat 1986) | |
| - | ** | + | *Cells grown to 10^9 cells/mL (Mermet-Bouvier) |
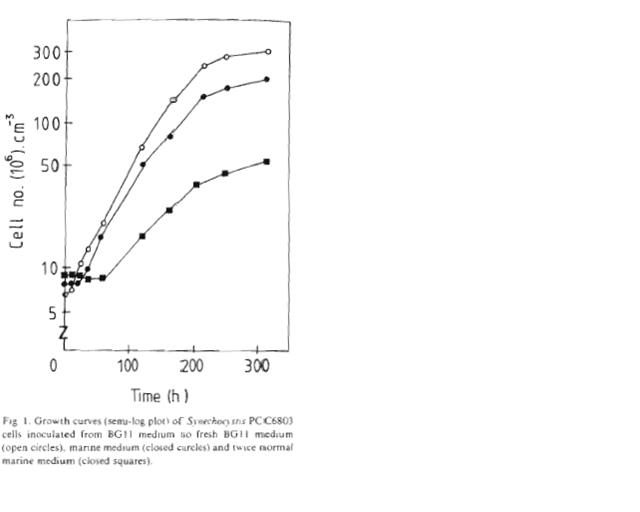
| + | **A [[Team:Hawaii/PCC6803 cell density| PCC6803 cell density]] test was performed and aligned with a growth curve (Richardson 1983). | ||
| + | [[Image:Growth_curve.JPG|right|thumbnail|300 px|Growth curve (semi-log plot) of ''Synechocystis'' sp. PCC6803 cells inoculated in BG-11 medium (open circles), marine medium (closed circles), and twice normal marine medium (closed boxes).]] | ||
| + | <strong>Plasmid Concentration</strong> | ||
| + | *Use estimates from gel found in [[Team:Hawaii/Large-Scale Preparation of Plasmid from E. coli| Large-Scale Preparation of Plasmid from E. coli]] | ||
| + | *Use calculation from UV spectrometric reading found in [[Team:Hawaii/Large-Scale Preparation of Plasmid from E. coli| Large-Scale Preparation of Plasmid from E. coli]] | ||
== Results == | == Results == | ||
| Line 31: | Line 36: | ||
*Ludwig, Alfred, “Transformation and gene replacement in the facultatively chemoheterotrophic, unicellular cyanobacterium Synechocystis sp. PCC6714 by electroporation,” Appl Microbiol Biotechnol (2008) 78:729–735 | *Ludwig, Alfred, “Transformation and gene replacement in the facultatively chemoheterotrophic, unicellular cyanobacterium Synechocystis sp. PCC6714 by electroporation,” Appl Microbiol Biotechnol (2008) 78:729–735 | ||
*Chauvat, F, "A host-vector system for gene cloning in the cyanobacterium ''Synechocystis'' PCC6803," Mol Gen Genet (1986) 204:185-191. | *Chauvat, F, "A host-vector system for gene cloning in the cyanobacterium ''Synechocystis'' PCC6803," Mol Gen Genet (1986) 204:185-191. | ||
| + | * Richardson, D.L., "''Synechocystis'' PCC6803: a euryhaline cyanobacterium," FEMS Microbiology Letters 18 (1983) 99-102. | ||
<blockquote>''Insanity is doing the same thing over and over again and expecting different results.'' - Albert Einstein</blockquote> | <blockquote>''Insanity is doing the same thing over and over again and expecting different results.'' - Albert Einstein</blockquote> | ||
{{Team:Hawaii/Footer}} | {{Team:Hawaii/Footer}} | ||
Latest revision as of 18:33, 30 June 2008
| Projects | Events | Resources | ||
|---|---|---|---|---|
| Sponsors | Experiments | Milestones | Protocols | |
| Notebook (t) | Meetings (t) |
Contents |
Electroporation of PCC6803
- This experiment can be used to introduce autonomously replicating plasmids to PCC6803.
Methods
- 50 mL linear phase Synechocystis at OD730 0.5 collected by centrifugation
- wash cells three times with 1mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer pH 7.5
- remove supernatant, resuspend in 100 uL of the remaining liquid by vortexing
- 60 uL of cell suspension mixed with 0.1-16 ug DNA (dissolved in H2O) and electroporated with Biorad gene pulser (25uF capacitor used, time constant varied buy changing resistors (100, 200, 400, & 600 OHMS for time constant 2.5, 4.8, 9, 13 ms, respectively, electric field varied from 0-12kV cm-1)
- IMMEDIATELY after electric pulse, cells resuspended in 1 mL BG11 & mixed with 50 mL BG11 in an Erlenmeyer flask
- cells incubated 5 days under **specified growth conditions
- 50 mL culture collected by centrifugation, resuspended in 500uL of remaining liquid, mixed with 5mL BG11 soft agar (BG11 + 0.75% agar)
- Pour on plates with 25 mL BG11 agar with antibiotic
- determine number of viable cells after electroporation: 10^-4 dilution made before collecting the culture, 50 uL of this dilution plated with out selection, incubate 14 days, count colonies. Total viable cells (colonies grown on non-selective plates * 10^7) and total number transformants (colonies on selective plates) determined
Cultivation
- Synechocystis is photo-autotrophically grown in BG11 at 30°C under white light (Chauvat 1986)
- Cells grown to 10^9 cells/mL (Mermet-Bouvier)
- A PCC6803 cell density test was performed and aligned with a growth curve (Richardson 1983).
Plasmid Concentration
- Use estimates from gel found in Large-Scale Preparation of Plasmid from E. coli
- Use calculation from UV spectrometric reading found in Large-Scale Preparation of Plasmid from E. coli
Results
Discussion
- 40uL of cells at 10^9 cells/mL were mixed with 5 uL of plasmid DNA solution (1ug/uL). Electroporation was performed at field strength of 12kV/cm at a constant of 5ms, with no cell death detected. (Mermet-Bouvier)*used for PCC6803 and other strains.
- This protocol is adapted from a protocol for PCC6714, which is not naturally competent. However it seems we can use this for PCC6803 so we can obtain autonomously replicating plasmids.
References
- Ludwig, Alfred, “Transformation and gene replacement in the facultatively chemoheterotrophic, unicellular cyanobacterium Synechocystis sp. PCC6714 by electroporation,” Appl Microbiol Biotechnol (2008) 78:729–735
- Chauvat, F, "A host-vector system for gene cloning in the cyanobacterium Synechocystis PCC6803," Mol Gen Genet (1986) 204:185-191.
- Richardson, D.L., "Synechocystis PCC6803: a euryhaline cyanobacterium," FEMS Microbiology Letters 18 (1983) 99-102.
Insanity is doing the same thing over and over again and expecting different results. - Albert Einstein
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"