Team:Princeton/ProjectDetails
From 2008.igem.org
(→The Toggle Switch) |
(→The Toggle Switch) |
||
| Line 3: | Line 3: | ||
{|align="center" | {|align="center" | ||
| - | |[[Image: | + | |[[Image:Toggleswitch5_Princeton.jpg|650px]] |
|} | |} | ||
Revision as of 02:13, 30 October 2008
PRINCETON IGEM 2008
| Home | Project Overview | Project Details | Experiments | Results | Notebook |
|---|
| Parts Submitted to the Registry | Modeling | The Team | Gallery |
|---|
Contents |
The Toggle Switch
|
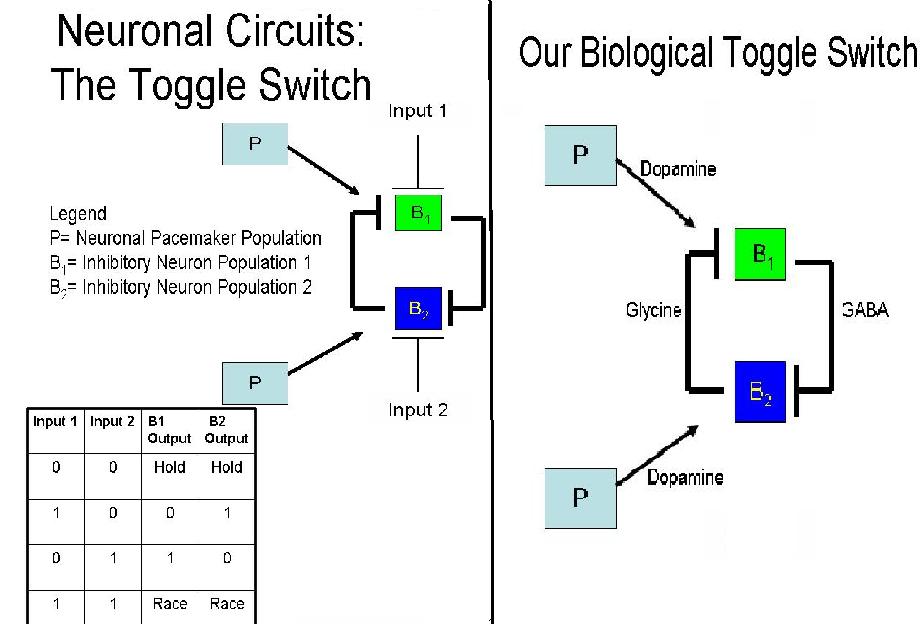
A toggle switch is a bistable system, where two inputs are responsible for two different outputs - similar to an on-off switch. The system depicted to the right consists of three groups of cells; one pacemaker group (P), and two groups of neurons (B1 and B2). The pacemaker cells are used as a source of continuous potentiation, consistently activating B1 and B2.
B1 and B2 continuously inhibit each other, which requires their signals to be able to overcome the excitation of the pacemaker cells. Continuous output from P, as well, results in the continuous inhibition from B1 and B2 for the ability to hold state. When Input1 is introduced into the system, B1 is inhibited, discontinuing the inhibition of B2. Thus, we have B2 as an output. Conversely, when Input 2 is applied, B2 is inhibited and B1 is the output.
If at any time both inputs were introduced into the system, the system would suffer a race condition. Over time, the strongest signal for inhibition would gain a slight advantage and one of the two conditions would achieve an output. For our purposes, however, one input will be introduced into the system so as to gain a singular output and test the functionality of our designed system.
The diagram to the right lists the neurotransmitters we have chosen to control the neuronal toggle switch. In this case, the excitatory signal produced by the pacemaker cells is a result of Dopamine, in combination with Dopamine Receptor 5 located on the B1 and B2 neurons. Their continued activation causes the production of Glycine by B2 cells and GABA by B1 cells, both of which are inhibitory neurotransmitters. This continuous signal from the pacemaker group, coupled with consistent inhibition creates a hold state between neurons. When there is more of one inhibitory neurotransmitter in the system, the opposite neuronal group will be inhibited and the other group will have an output.

|
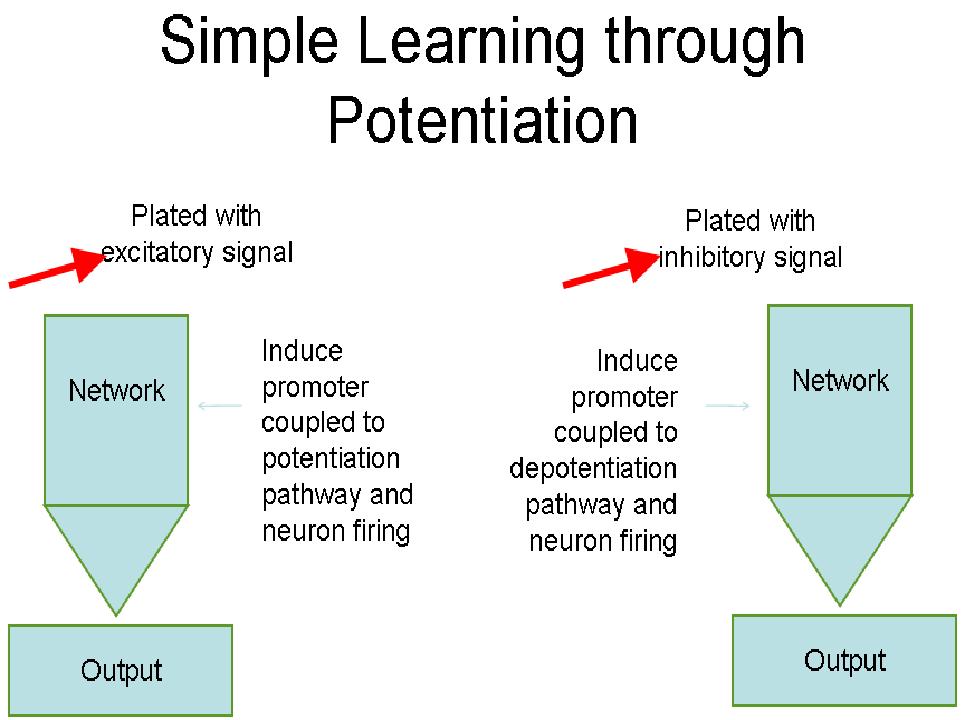
The Learning Circuit
|
|
Plasmid Designs
We worked on designing and constructing several plasmids this summer. Our plasmids were on the whole designed using PCR SOEing (Splicing by Overlap Extension). The process involves PCRing the pieces of the plasmid insert separately, but adding one or more "overlap" regions to each insert section that consists of the end sequence of the neighboring section.
For example, if the insert to be put into the vector is A-B-C, then A would be PCRed with a bit of the beginning of B added onto the end using a primer overhang. B would be PCRed with a bit of the beginning of C added on (the end of A need not be added onto the beginning of B because B already has an overlap with the beginning of its sequence that was added onto the end of A). Then A could be "SOEed" to B by running a PCR reaction of equimolar amounts of A and B, the forward primer of A and the reverse primer of B. A and B will anneal to each other and acts as primers in addition to the oligos in the reaction. B and C could similarly be SOEed, followed by A-B and B-C. A and B and C could also be SOEed together in a single reaction, with every piece acting as primer, but there are decreasing outputs the more pieces that one attempts to SOE in a single reaction.
A list of plasmids we have designed and worked on is given here, each linking to a design page. Some plasmids were redesigned, and hence appear more than once, with a number tacked on the end. At the top of each page the name of the plasmid is listed, followed by the vector, with the position and names of the restriction sites used to cut, and the insert, similarly marked with restriction sites. If the enzymes do not match it they have compatible overhangs.
PCR SOEing and Sequencing Primers
We created over 100 primers for the construction and sequencing of our plasmids. For the sequences and properties of each primer, as well as which primers were used for which plasmids, click here.
Surface Patterning
Previous Work I - Preparation of Gold Surface for Cell Adhesion
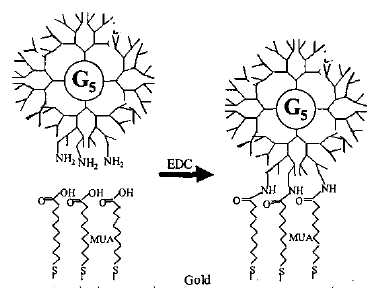
M.B. Shenai et al (2003) have demonstrated a protocol for viable cell adhesion. A layer of chrome or titanium is deposited on a glass slide, followed by the deposition of a layer of gold by electron beam evaporation in the required pattern. Once this surface has been cleaned of organic impurities, it is covered by a 7.5M solution of mercaptoundecanoic acid (MUA), which adheres to the gold and has carboxyl groups on the other end. These groups are activated (using a carbodiimide) and they attach to the amide groups in a synthetic polymer called a dendrimer that forms the final layer on top of the gold. The image below shows a cross-section of the surface once it is ready for the addition of medium and cell adhesion to the dendrimer.
|
The MUA is attached to the gold, while the EDC activates the carboxyl groups of the MUA to attach to the amide groups of the dendrimer.
Previous Work II - Patterning of the Gold Surface
It has been shown that plating neurons in defined patterns gives the emerging neural networks properties that are directly related to those patterns. Assaf Rotem of the Weizmann Institute demonstrated that triangular plating, for example, encourages the propagation of asymmetric signals and directed axonal growth, thereby acting somewhat similar to a diode.
|
References
Nanotexturing Gold Surfaces with Dendrimers for Selective Cell Adhesion M.B. Shenai et. al; 2003
Reliable neuronal logic devices from patterned hippocampal cultures (Under Review) Feinerman, Rotem and Moses; 2008
 "
"