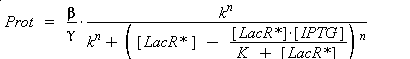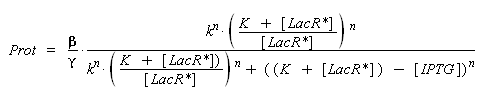Team:Paris/Modeling/estimation
From 2008.igem.org
(→first hypothesis) |
|||
| Line 44: | Line 44: | ||
===how to control the concentration of the transcription factor ?=== | ===how to control the concentration of the transcription factor ?=== | ||
| - | Now, we must use as a variable of reference an element that could be introduced in the bacteria, well-controlled, and from which will depends all the concentrations of our transcription factor. We propose a construction in which our transcription factor is put after the promoter ''Plac'', which is under the repression of | + | Now, we must use as a variable of reference an element that could be introduced in the bacteria, well-controlled, and from which will depends all the concentrations of our transcription factor. We propose a construction in which our transcription factor is put after the promoter ''Plac'', which is under the repression of LacR. Since IPTG is a small diffusive molecule that binds to LacR and inhibits this way the repression of ''Plac'', we can use it as an 'inducer'. To do so, we must place in the bacterium the gene ''lacR'' after a constitutive promoter (like J23101). According to previous hypothesis, this will provide at steady-state a 'constant concentration' of LacR (we note [LacR*], and it is supposed to be the TOTAL concentration of LacR, under every form) in the bacterium. If we consider the binding reaction this way (where LacR_IPTG denotes the complex) |
| - | <center> | + | <center> LacR + IPTG ⇄ LacR_IPTG </center> |
with a dissociation constant K, we find at the steady-state | with a dissociation constant K, we find at the steady-state | ||
| - | <center> [[Image: | + | <center> [[Image:Bilan_lacR.png]]</center> |
| - | where [IPTG] denotes the concentration of IPTG we introduced in the medium, that will stay constant in all the bacteria along time, assuming that its degradation is near 0, and that the diffusion is quick. (We can already notice an other limitation in our protocol : this formula has got sense only for [IPTG] < (K + [ | + | where [IPTG] denotes the concentration of IPTG we introduced in the medium, that will stay constant in all the bacteria along time, assuming that its degradation is near 0, and that the diffusion is quick. (We can already notice an other limitation in our protocol : this formula has got sense only for [IPTG] < (K + [LacR*]), that limits the range of [IPTG] we can use to determine the functions below. Notice that this limitation is conditionned by [LacR*], which depends of the constitutive promoter we put before ''lacR''.) |
According to the [[Team:Paris/Modeling#first_Hypothesis|hypothesis '''(3)''']], the activity of ''Plac'' would verify (keeping the same notations) : | According to the [[Team:Paris/Modeling#first_Hypothesis|hypothesis '''(3)''']], the activity of ''Plac'' would verify (keeping the same notations) : | ||
| - | <center> [[Image: | + | <center> [[Image:act_plac.png]]</center> |
| - | so, if k denotes the dissociation constant of ( | + | so, if k denotes the dissociation constant of (LacR + ''Plac'' ⇄ LacR_''Plac''), |
| - | <center> [[Image: | + | <center> [[Image:act_plac_dvlp.png]] </center> |
In the last equation, we will have 'access' (see [[Team:Paris/Modeling#first_Hypothesis|hypothesis '''(5)''']] | In the last equation, we will have 'access' (see [[Team:Paris/Modeling#first_Hypothesis|hypothesis '''(5)''']] | ||
| - | ) to Prot and possibly to gamma, and we are looking for beta, k and n, thanks to our program (see [[Team:Paris/Modeling#getting_a_Hill_function_from_convenient_datas|getting Hill function with convenient datas]]). But we need to know ([ | + | ) to Prot and possibly to gamma, and we are looking for beta, k and n, thanks to our program (see [[Team:Paris/Modeling#getting_a_Hill_function_from_convenient_datas|getting Hill function with convenient datas]]). But we need to know ([LacR*] - [LacR*].[IPTG]/(K + [LacR*])), and we just have [IPTG]. However, we can reduce the last equation to |
| - | <center> [[Image: | + | <center> [[Image:equ_redu.png]]</center> |
Thus, we have two possibilities : | Thus, we have two possibilities : | ||
* we can write a new algorithm that optimise an approaching solution of the new parameters, based on the same principal than [[Team:Paris/Modeling/Programs|'findparam']]. | * we can write a new algorithm that optimise an approaching solution of the new parameters, based on the same principal than [[Team:Paris/Modeling/Programs|'findparam']]. | ||
| - | * better but much longer and requiring much more precision, we can use the already noticed properties : [IPTG] < (K + [ | + | * better but much longer and requiring much more precision, we can use the already noticed properties : [IPTG] < (K + [LacR*]). By having a look on the first equation of this section, we understand that beyond this limit, [LacR_IPTG] will no more evoluate. By observing the evolution of the influence of a growing (by steps as small as possible) concentration of [IPTG] introduced, we should be able to approximate the critic concentration when it no more changes, ~(K + [LacR*]). Less is the order n, better is the detection of this critic concentration, because of the greater derivative of the Hill function for small values of LacR. Therefore we should keep this estimation only if we find n ~ 1. Then, by considering k*((K + [LacR*])/[LacR*]) instead of k, we should easily determine all the parameters we need, only thanks to [[Team:Paris/Modeling/Programs|'findparam']]. |
===what are we looking for ?=== | ===what are we looking for ?=== | ||
Revision as of 18:19, 12 August 2008
|
 "
"