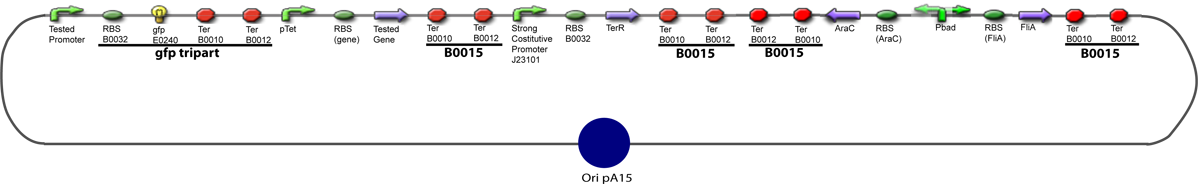
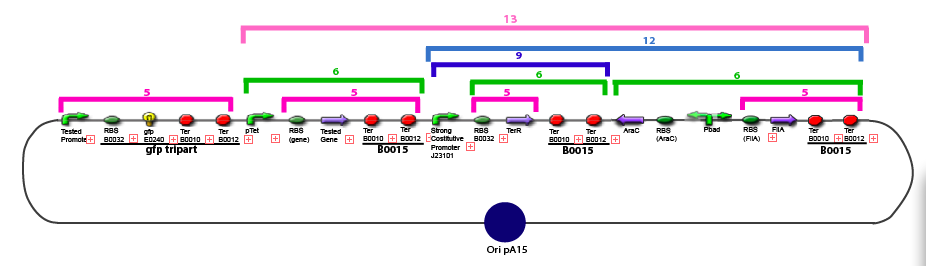
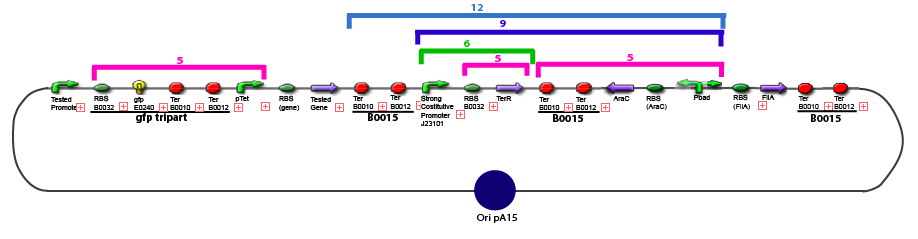
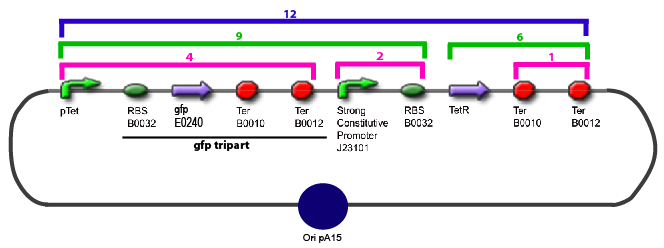
Team:Paris/Notebook/Protocols
From 2008.igem.org
| Line 1: | Line 1: | ||
{{Paris/Menu}} | {{Paris/Menu}} | ||
| - | + | = Culture of Stable strain with biobricks 2008 = | |
* Streaks on plates with LB and the adapted antibiotics to isolate colonies | * Streaks on plates with LB and the adapted antibiotics to isolate colonies | ||
* Incubate O/N at 37°C | * Incubate O/N at 37°C | ||
| - | |||
* Take clone with a toothpick and put in 7.5ml LB with adaptated antibiotics (LB have to be prepared before !!!) | * Take clone with a toothpick and put in 7.5ml LB with adaptated antibiotics (LB have to be prepared before !!!) | ||
* Will be use for Miniprep and Stock in glycerol | * Will be use for Miniprep and Stock in glycerol | ||
* 2-3 clones isolated by Biobricks | * 2-3 clones isolated by Biobricks | ||
* Incubate O/N at 37°C (open just a little the cap for bacteria's breathing) | * Incubate O/N at 37°C (open just a little the cap for bacteria's breathing) | ||
| - | |||
*Don't store LB in the fridge even with antibiotics ! | *Don't store LB in the fridge even with antibiotics ! | ||
| - | + | = Glycerol Stocks = | |
* Remove 2.5mL of each culture and centrifuge. | * Remove 2.5mL of each culture and centrifuge. | ||
* Discard the supernatant and resuspend pelleted bacteria in 1mL of LB. | * Discard the supernatant and resuspend pelleted bacteria in 1mL of LB. | ||
| Line 19: | Line 17: | ||
* Store at -20°C. | * Store at -20°C. | ||
| - | + | = Minipreps (Kit Qiagen) = | |
* centrifuge 5mL of culture 8 min at 3,500 to 4,000 g. | * centrifuge 5mL of culture 8 min at 3,500 to 4,000 g. | ||
* Resuspend pelleted bacterial cells in 250 µl Buffer P1 and transfer to a microcentrifuge tube. | * Resuspend pelleted bacterial cells in 250 µl Buffer P1 and transfer to a microcentrifuge tube. | ||
| Line 32: | Line 30: | ||
* To elute DNA, place the QIAprep column in a clean 1.5 ml microcentrifuge tube. Add 30 µl Buffer EB or water to the center of each QIAprep spin column, let stand for 1 min, and centrifuge for 1 min. | * To elute DNA, place the QIAprep column in a clean 1.5 ml microcentrifuge tube. Add 30 µl Buffer EB or water to the center of each QIAprep spin column, let stand for 1 min, and centrifuge for 1 min. | ||
| - | + | = Electrophoresis = | |
| - | + | ||
An electrophoresis can be done to check if there is Product of Miniprep | An electrophoresis can be done to check if there is Product of Miniprep | ||
* Gel : 1% agarose with BET added (5 µL BET for 100 mL TBE) | * Gel : 1% agarose with BET added (5 µL BET for 100 mL TBE) | ||
| Line 40: | Line 37: | ||
| - | + | = Concentration of the Miniprep = | |
By biophotometry | By biophotometry | ||
* Blank : 55 µL of pure water + 5 µL EB | * Blank : 55 µL of pure water + 5 µL EB | ||
* Sample : 55 µL of pure water + 5 µL of DNA | * Sample : 55 µL of pure water + 5 µL of DNA | ||
| - | Check if the ratio 260/280 is over 1,6 | + | Check if the ratio 260/280 is over 1,6 |
| - | + | '''Think about the dilution !''' | |
| + | = Digestion = | ||
* 1 µg of plasmid / 250 ng of gene | * 1 µg of plasmid / 250 ng of gene | ||
* Buffer (n°2) 10X : 3µL | * Buffer (n°2) 10X : 3µL | ||
| Line 54: | Line 52: | ||
* Pure water qsp 30 µL | * Pure water qsp 30 µL | ||
* 1 µL of each enzyme | * 1 µL of each enzyme | ||
| - | |||
* Incubate during about 2h30-3h at 37°C, then 20 minutes at 65°C (to inactivate the enzymes). | * Incubate during about 2h30-3h at 37°C, then 20 minutes at 65°C (to inactivate the enzymes). | ||
| - | + | = Migration after digestion = | |
| - | + | ||
* Run the whole samples (30 µL) in a '''0.8-1% agarose gel''' (a new one) | * Run the whole samples (30 µL) in a '''0.8-1% agarose gel''' (a new one) | ||
* Run at 50 V until halfway | * Run at 50 V until halfway | ||
| Line 64: | Line 60: | ||
* 30 µL of DNA + 6 µL of LB | * 30 µL of DNA + 6 µL of LB | ||
| - | + | '''Separate each band by an empty one !''' | |
| - | + | ||
| - | + | ||
| + | = Extraction = | ||
* For each new extraction it's important to have a new bath of ETB | * For each new extraction it's important to have a new bath of ETB | ||
* Use a new blade for each extraction | * Use a new blade for each extraction | ||
* The band weight must be less than 200 mg | * The band weight must be less than 200 mg | ||
| - | + | = Amplification of promoters = | |
=>To amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.'' | =>To amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.'' | ||
* '''Preparation of the templates''' : Resuspend of 1 colony in 100µl of water. | * '''Preparation of the templates''' : Resuspend of 1 colony in 100µl of water. | ||
Revision as of 11:42, 28 August 2008
Culture of Stable strain with biobricks 2008
Glycerol Stocks
Minipreps (Kit Qiagen)
ElectrophoresisAn electrophoresis can be done to check if there is Product of Miniprep
Concentration of the MiniprepBy biophotometry
Check if the ratio 260/280 is over 1,6 Think about the dilution ! Digestion
Migration after digestion
Separate each band by an empty one ! Extraction
Amplification of promoters=>To amplify the sequence in order to have enough amount of DNA to carry out the following of our experiments.
For each samples,
1 µl dNTP
LID : 105°C Purification (Kit Promega)Gel Slice and PCR Product PreparationDissolving the Gel Slice
Processing PCR reactionsFor products above 40 pb
Binding of DNA
Washing
Elution
Quantification by electrophoresis
Ligation
TransformationUse of TOP10 chemically competent cells
PCR ScreeningUse of 8 clones of Ligation transformants for screening PCR
After, add
Store the tubes on ice waiting for PCR attains 95°C then put the tubes in the machine
LID 105°C
Electrophoresis Purification of PCR
Sequencingvoir ici -> [http://institut.cochin.inserm.fr/rubric_recherche/Plates-Formes/sequencage_genomique/I18NFolder.2005-02-10.4781618697/page2/fr Sequencing COCHIN]
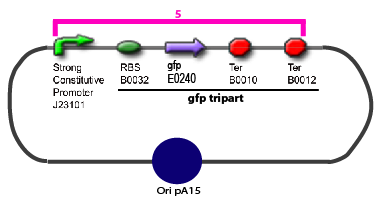
Promoter Characterization PlanFor theoretical consideration, see estimation of parameters
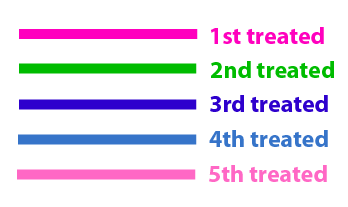
The same colour coded steps can be perfomed at the same time if elements needed are available The order for treating the colours should of course be:
This table contains the promoters we need to characterize, the transcription factors whose effect on the promoter we want to test, and the plasmid we want to obtain in order to carry out each characterization
Protocol to make competent bacteria
Before: prepare CaCl2 0.1M.
Study of the doubling time of the bacteria population
- B1 Vitamin 0.1%
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"