Team:Heidelberg/Notebook/Sensing Group/Cloning
From 2008.igem.org
(→LuxP) |
(→Fusion receptor) |
||
| Line 484: | Line 484: | ||
=== Fusion receptor === | === Fusion receptor === | ||
| - | A detailed description of the Tar receptor and LuxQ quorum-sensing receptor can be found in the [[Team:Heidelberg/Project/Sensing | project description]]. Since it is believed that the linker region (transmembrane domain TM2) is | + | A detailed description of the Tar receptor and LuxQ quorum-sensing receptor can be found in the [[Team:Heidelberg/Project/Sensing | project description]]. Since it is believed that the linker region (transmembrane domain TM2) is critical for signal transduction, two different receptor constructs will be designed, referred to as Fusion-1 and Fusion-2. Fusion-1 will contain the cytoplasmic domain of the Tar receptor and the sequence of the second transmembrane domain,TM2 up to the very N-terminus of LuxQ (TM2, periplasmic domain, TM1). Fusion-2 will contain the cytoplasmic domain as well as the second transmembrane domain, TM2 of Tar followed by the periplasmic domain up to the N-terminal sequence end of LuxQ (periplasmic domain, TM1). |
| - | The receptor chimeras will be | + | The receptor chimeras will be generated in two sequential PCR reactions (Fusion PCR). In the first PCR the two single fragments containing parts of the coding sequence of LuxQ and Tar will be amplified. The reverse primer for LuxQ and the forward primer for Tar part have complementary ends allowing annealing during the second PCR reaction. In addition to the amplified sequences from the first reaction the forward primer for LuxQ and the reverse primer for Tar will be added to the reaction mix after the gel purification in order to be able to amplify the chimeric receptor sequence. |
=== References === | === References === | ||
[1] Dong, X.; Stothard, P.; Forsythe, I. J. & Wishart, D. S., '''PlasMapper: a web server for drawing and auto-annotating plasmid maps''', Nucleic Acids Res, Vol. 32, pp. W660-W664, 2004 | [1] Dong, X.; Stothard, P.; Forsythe, I. J. & Wishart, D. S., '''PlasMapper: a web server for drawing and auto-annotating plasmid maps''', Nucleic Acids Res, Vol. 32, pp. W660-W664, 2004 | ||
Revision as of 19:11, 27 October 2008


Contents |
Sensing - Cloning strategy
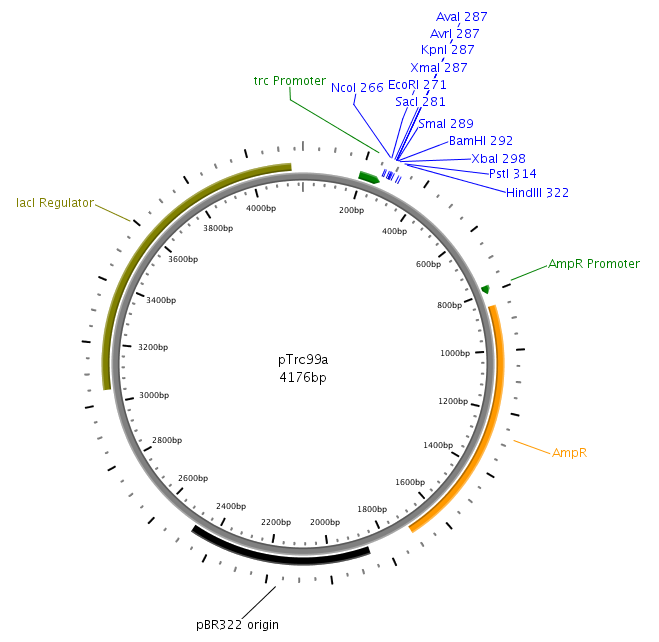
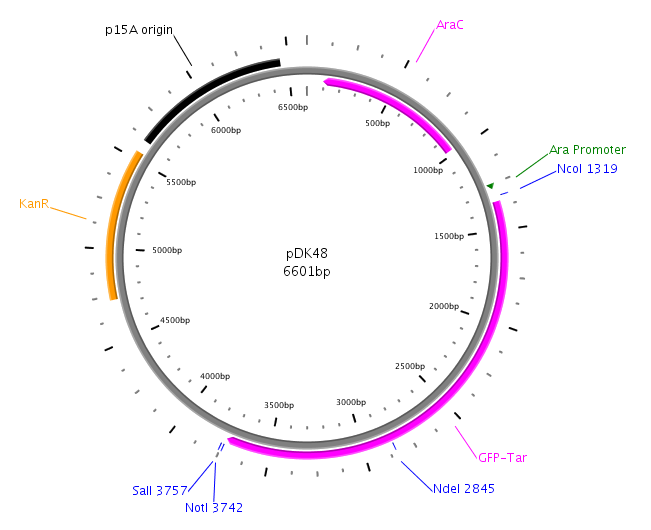
The core part of the sensing project is the construction of the LuxQ-Tar chimeric receptor, which enables the E. coli killer bacteria to chemotactically respond to a Autoinducer 2 (AI-2) gradient and detect prey cells. The quorum-sensing system will be amplified from the V. harveyi genome while the Tar receptor is from E. coli. On one hand LuxS needs to be cloned and transformed into one cell type, in order to make them produce and secrete AI-2. On the other hand the periplasmic ligand binding domain of LuxQ will be fused with the cytoplasmic domain of Tar and cloned on one plasmid together with LuxP which is necessary for AI-2 binding. Generally, restriction sites required for cloning are introduced via the PCR primer. In silico cloning was performed in [http://serialbasics.free.fr/Serial_Cloner.html SerialCloner], Vector maps were designed with PlasMapper [1].
LuxS
LuxS will be amplified from the Vibrio harveyi genome using the following primers: LuxSa and LuxSb. The complete sequence of the used primers can be found in tableXXX. Subsequently, the product will be cloned into the plasmid pTr99alpha using the NcoI and BamHI restriction sites and then transformed into DH5alpha competent cells.
LuxQ
LuxQ will be amplfied with primers LuxQa (forward) and LuxQc (reverse) from the V. harveyi genome. The obtained fragment will then be cloned into the plasmid pTrc99alpha using the NcoI and BamHI restriction sites. Subsequently, the product will be used as a template for PCR amplification with respect to cloning of the chimeric receptors (see below).
LuxP
We want to amplify LuxP by PCR using the following primers: LuxPc (forward) and LuxPd (reverse) from the V. harveyi genome. The PCR product will then be cloned into the vector pDK48 containing the LuxQ-Tar chimeric receptor sequence. This will be done using the restriction sites SalI and NotI. The resulting expression plasmid will be used for the transformation of our killer cells.
Fusion receptor
A detailed description of the Tar receptor and LuxQ quorum-sensing receptor can be found in the project description. Since it is believed that the linker region (transmembrane domain TM2) is critical for signal transduction, two different receptor constructs will be designed, referred to as Fusion-1 and Fusion-2. Fusion-1 will contain the cytoplasmic domain of the Tar receptor and the sequence of the second transmembrane domain,TM2 up to the very N-terminus of LuxQ (TM2, periplasmic domain, TM1). Fusion-2 will contain the cytoplasmic domain as well as the second transmembrane domain, TM2 of Tar followed by the periplasmic domain up to the N-terminal sequence end of LuxQ (periplasmic domain, TM1).
The receptor chimeras will be generated in two sequential PCR reactions (Fusion PCR). In the first PCR the two single fragments containing parts of the coding sequence of LuxQ and Tar will be amplified. The reverse primer for LuxQ and the forward primer for Tar part have complementary ends allowing annealing during the second PCR reaction. In addition to the amplified sequences from the first reaction the forward primer for LuxQ and the reverse primer for Tar will be added to the reaction mix after the gel purification in order to be able to amplify the chimeric receptor sequence.
References
[1] Dong, X.; Stothard, P.; Forsythe, I. J. & Wishart, D. S., PlasMapper: a web server for drawing and auto-annotating plasmid maps, Nucleic Acids Res, Vol. 32, pp. W660-W664, 2004
 "
"