Team:Heidelberg/Notebook/Killing I/Notebook/week11
From 2008.igem.org
(Difference between revisions)
(→Tuesday, 10/14/08) |
(→Wednesday, 10/15/08) |
||
| Line 609: | Line 609: | ||
*Gel purification kit | *Gel purification kit | ||
| - | + | ===Characterization of oriT=== | |
| + | *Quantitatively test for oriT<br> | ||
| + | Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 2.08<br> | ||
| + | Recipient: overnight culture Top10 pBAD 33 OD(600nm): 2.24<br> | ||
| + | **Centrifuge 350ul overnight culture in 1.5ml eppi for 2min at 13000rpm, 8samples donor, 8samples recipient | ||
| + | **Wash the pellet twice with LB medium | ||
| + | **Resolve the pellet in 350ul LB medium | ||
| + | **Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid | ||
| + | **Add the washed donor suspension | ||
| + | **Vortex and resolve the pellet | ||
| + | **Centrifuge the mix for 1min at 13000rpm | ||
| + | **Resolve the pellet in 100ul LB | ||
| + | **Put membrane filter on the LB agar | ||
| + | **Pipett the suspension on membrane filter (8samples) | ||
| + | **Incubate the plates with membrane filter at 37°C | ||
| + | **Put directly one membrane filter into 1ml LB in an 1.5ml eppi | ||
| + | **Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min) | ||
| + | **After 6, 12, 18, 24, 30, 36, 42 min repeat the last two steps. | ||
| + | **Negative control plates: | ||
| + | ***LB/Cm+Amp: | ||
| + | <br>100ul donor overnight culture<br> | ||
| + | 100ul recipient overnight culture<br> | ||
| + | **Cell number determination | ||
| + | ***LB/Cm: 100ul 10-6 recipient overnight culture | ||
| + | ***LB/Kan+Amp: 100ul 10-6 donor overnight culture | ||
| + | <br> | ||
| + | *Result: | ||
| + | **Negative control: negative | ||
| + | **Colony on LB/Cm: 238 (Titer of recipient: 2.38e9/ml) | ||
| + | **Colony on LB/Kan+Amp: 99 (Titer of donor: 0.99e9/ml) | ||
| + | **Colony on other LB/Cm+Amp plates: | ||
| + | ***10-5 dilute: <br> | ||
| + | Time Colony<br> | ||
| + | 0 9<br> | ||
| + | 6 20<br> | ||
| + | 12 94<br> | ||
| + | 18 172<br> | ||
| + | 24 262<br> | ||
| + | <br> | ||
| + | ***10-6 dilute:<br> | ||
| + | Time Colony<br> | ||
| + | 30 145<br> | ||
| + | 36 179<br> | ||
| + | 42 217<br> | ||
| + | <br> | ||
| + | *Inoculate the cells for conjugation test<br> | ||
| + | 5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33<br> | ||
| + | 5ml LB/chloramphenicol + 1colony MG1655 pBAD 33<br> | ||
| + | 5ml LB/chloramphenicol + 1 colony stock DH5alpha pBAD 33<br> | ||
| + | 15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307<br> | ||
==Thursday, 10/16/08== | ==Thursday, 10/16/08== | ||
Revision as of 13:05, 29 October 2008


| << Week 10 | Overview | Week 12 >> |
|---|
Week 11
Contents |
Monday, 10/13/08
Proceeding of phage cloning strategy two
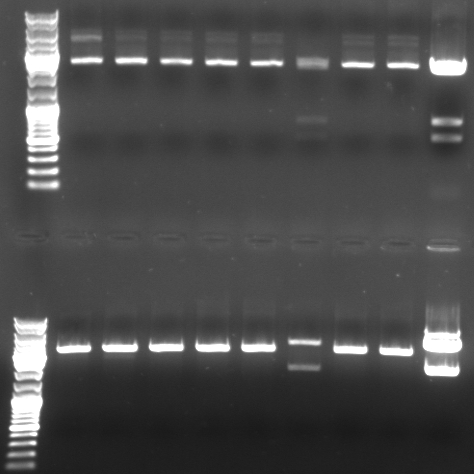
- Digestion of the miniprep 1-6,9,10 from sunday and the original pBluescript with insert
- Digestion with XbaI/XhoI (top)
- normal: 4549, 2157, 34
- new: 3945, 2898
- Digestion with SacI/SpeI (bottom)
- normal: 4549, 2157, 34
- new: 4549, 1331, 929, 34
- Gel
- lane0: dna ladder mix
- lane1: sample 1
- lane2: sample 2
- lane3: sample 3
- lane4: sample 4
- lane5: sample 5
- lane6: sample 6
- lane9: sample 9
- lane10: sample 10
- lane11: pBluescript with insert
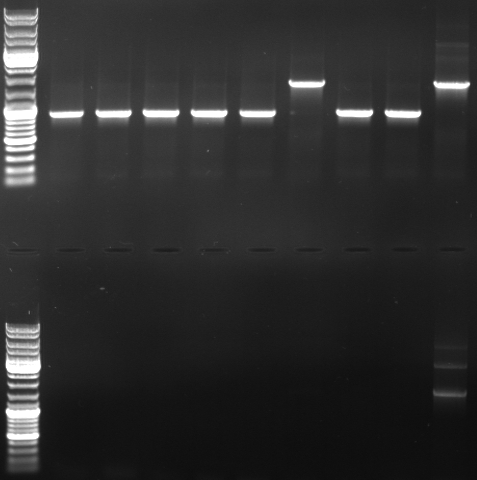
- PCR with CmR_suffix and CmR_prefix (top)
- normal: 1668bp
- new: 852bp, 919bp
- PCR with oriT_prefix and CmR_suffix (bottom)
- normal: 2150bp
- new: 1329bp
- Gel
- lane0: dna ladder mix
- lane1: sample 1
- lane2: sample 2
- lane3: sample 3
- lane4: sample 4
- lane5: sample 5
- lane6: sample 6
- lane9: sample 9
- lane10: sample 10
- lane11: pBluescript with insert
- --> we do not have a working GFP/CmR in pBlue/insert!!! --> do the ligation again (beginng from the mutagenesis pcr)
Proceeding of cloning CmR and oriT in standard plasmid
- inoculation of CmR Std. Mutagenesis PCR sample and oriT Std. colonies
Tuesday, 10/14/08
Proceeding of cloning CmR and oriT in standard plasmid
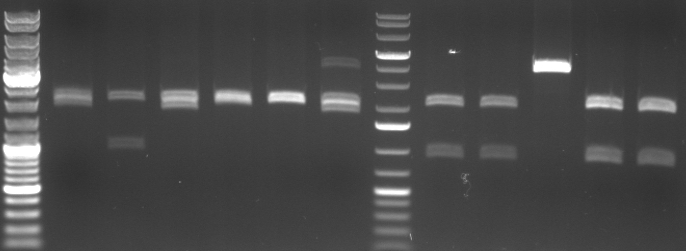
- Miniprep of 6 oriT and 5 CmR Std. samples
- digestion with EcoRI/PstI (to cut out insert of pSB1A2)
- lane0: dna ladder mix
- lane1-6: oriT 1-6
- lane7: 1kb dna ladder plus
- lane8: CmR Std. Mut 1.1.1
- lane9: CmR Std. Mut 1.1.2
- lane10: CmR Std. Mut 1.2.2
- lane11: CmR Std. Mut 2.2.1
- lane12: CmR Std. Mut 2.2.2
- expected fragments:
- oriT: 2000bp, 500bp
- CmR: 2000bp, ca. 900bp
- expected fragments:
- -->no oriT is right
- -->CmR 1.1.1, 1.1.2, 2.2.1, 2.2.2 look good
- -->sequencing of 2.2.1 and 2.2.2
- sequencing results match perfect
- Three ligations of oriT pcr product with pSB1A2 backbone (PstI, XbaI) (1:2.5, 2:5, 3:7.5 (µl, vector:insert))
- 40min at RT
- transformation, plated out on Amp plates
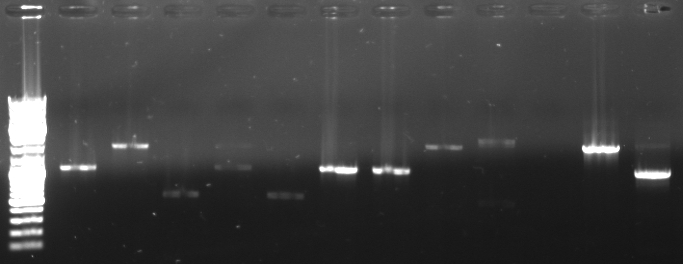
- Screening PCR of oriT agar plates
- pcr with standard plasmid primers (VF2, VR) using Taq
- 12 pcr samples (1-6 only one colony, 7-12 three colonies)
- Gel
- lane0: dna ladder mix
- lane1-12: screening sample 1-12
- expected fragment length: ca. 500-600bp
- -->sample 3 and 5 look good
- -->inoculation of overnight cultures
- -->sequencing of 3 and 5
- sequencing results match perfect
Phage cloning strategy two
- Mutagenesis PCR of pBlue with insert to remove KpnI restriction site
- using turbo Pfu
- elongation: 12,5min
- DpnI digestion 2h at 37°C
- transformation in TOP10, plated out on Cm plate
Phage cloning strategy one
- mutation of old insert in pBluescript
- pBluescript + insert was cut with BamHI --> the resulting backbone cut out of the gel and religated. With this vector 2 mutagenesis PCRs were done to eleminate the two remaining XbaI restriction sites.
- The resulting vector was digested with XbaI/XhoI to get the insert with the correct restriction sites for ligation into lambda phage
Characterization of oriT
- Inoculate the cells for conjugation test
5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33
5ml LB/kanamycin/ampicilin +glycerol stock Top10 J01103+pUB307
Wednesday, 10/15/08
Phage cloning strategy two
- inoculation of KpnI mutagenesis PCR samples --> Miniprep
- Digestion with KpnI/AgeI
- Gel
- Gel purification kit
Characterization of oriT
- Quantitatively test for oriT
Donor: overnight culture Top10 oriT+pUB307 OD(600nm): 2.08
Recipient: overnight culture Top10 pBAD 33 OD(600nm): 2.24
- Centrifuge 350ul overnight culture in 1.5ml eppi for 2min at 13000rpm, 8samples donor, 8samples recipient
- Wash the pellet twice with LB medium
- Resolve the pellet in 350ul LB medium
- Centrifuge the washed recipient for 2min at 13000rpm, discard the fluid
- Add the washed donor suspension
- Vortex and resolve the pellet
- Centrifuge the mix for 1min at 13000rpm
- Resolve the pellet in 100ul LB
- Put membrane filter on the LB agar
- Pipett the suspension on membrane filter (8samples)
- Incubate the plates with membrane filter at 37°C
- Put directly one membrane filter into 1ml LB in an 1.5ml eppi
- Vortex the eppi for 30sec, dilute for 10-5 and 10-6, plate them out on LB/Amp+Cm plates (0min)
- After 6, 12, 18, 24, 30, 36, 42 min repeat the last two steps.
- Negative control plates:
- LB/Cm+Amp:
100ul donor overnight culture
100ul recipient overnight culture
- Cell number determination
- LB/Cm: 100ul 10-6 recipient overnight culture
- LB/Kan+Amp: 100ul 10-6 donor overnight culture
- Cell number determination
- Result:
- Negative control: negative
- Colony on LB/Cm: 238 (Titer of recipient: 2.38e9/ml)
- Colony on LB/Kan+Amp: 99 (Titer of donor: 0.99e9/ml)
- Colony on other LB/Cm+Amp plates:
- 10-5 dilute:
- 10-5 dilute:
Time Colony
0 9
6 20
12 94
18 172
24 262
- 10-6 dilute:
- 10-6 dilute:
Time Colony
30 145
36 179
42 217
- Inoculate the cells for conjugation test
5ml LB/chloramphenicol + glycerol stock Top10 pBAD 33
5ml LB/chloramphenicol + 1colony MG1655 pBAD 33
5ml LB/chloramphenicol + 1 colony stock DH5alpha pBAD 33
15ml LB/kanamycin/ampicilin +glycerol stock Top10 oriT+pUB307
Thursday, 10/16/08
Phage cloning strategy two
- overnight ligation of pBluescript/insert backbone, GFP and CmR
Friday, 10/17/08
Phage cloning strategy two
- transformation of overnight ligations in TOP10
Saturday, 10/18/08
Phage cloning strategy two
- inoculation of colonies from the transformation
Sunday, 10/19/08
Phage cloning strategy two
- Miniprep
| << Week 10 | Overview | Week 12 >> |
|---|
 "
"