Team:Brown/Project/Analysis
From 2008.igem.org
NeilParikh (Talk | contribs) (→NaCl Resistance Testing) |
|||
| Line 172: | Line 172: | ||
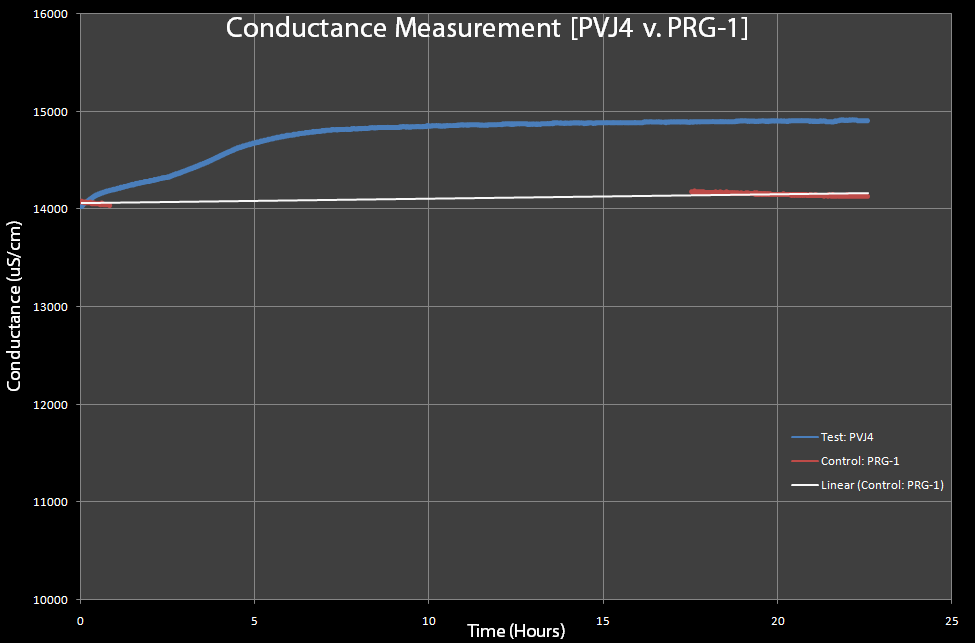
[[Image:Conductance-pvj4-v-prg1.gif|right|thumb|600px|Figure 8: This is a test of PVJ4 (which has the lysis cassette) and PRG-1 (which does not have the lysis cassette), with 0.2% Arabinose added to both. The PRG-1's conductance was measured before and after an overnight sitting and PVJ4 was measured continuously, due to limited equipment.]] | [[Image:Conductance-pvj4-v-prg1.gif|right|thumb|600px|Figure 8: This is a test of PVJ4 (which has the lysis cassette) and PRG-1 (which does not have the lysis cassette), with 0.2% Arabinose added to both. The PRG-1's conductance was measured before and after an overnight sitting and PVJ4 was measured continuously, due to limited equipment.]] | ||
| + | |||
| + | |||
* Different from expected resistance measurements, the conductivity of the lysed solutions greatly increased. Initial conductivity tests were run one after the other due to the limited availability of conductivity probes. Team Toxipop now has access to two probes. The probe was sensitive enough to pick up changes during cell lysis. The team did not need to concentrate the cells. Ten milliliter cultures were used only because the entire sensor (graphite) needed to be submerged within the solution. In future tests, we would like to minimize the volume of the bacterial solution to a fraction of a milliliter. Figure 7 is from tests run with the pVJ4 cassette with Arabinose added. The curve was what we expected to see. In addition, the increase in conductivity mirrors our Optical Density measurements with regard to time. Conductance increased after two to three hours while Optical Density decreased within that same time. Figure 8 represents a pVJ4 culture as well as the pRG1 plasmid (under the ''lacI'' promoter). Initial and final conductance readings were taken. Continuous readings could not be taken since the team only had access to one conductivity probe at the time. Nevertheless, the control remained the same before and after lysis had occurred in the experimental. | * Different from expected resistance measurements, the conductivity of the lysed solutions greatly increased. Initial conductivity tests were run one after the other due to the limited availability of conductivity probes. Team Toxipop now has access to two probes. The probe was sensitive enough to pick up changes during cell lysis. The team did not need to concentrate the cells. Ten milliliter cultures were used only because the entire sensor (graphite) needed to be submerged within the solution. In future tests, we would like to minimize the volume of the bacterial solution to a fraction of a milliliter. Figure 7 is from tests run with the pVJ4 cassette with Arabinose added. The curve was what we expected to see. In addition, the increase in conductivity mirrors our Optical Density measurements with regard to time. Conductance increased after two to three hours while Optical Density decreased within that same time. Figure 8 represents a pVJ4 culture as well as the pRG1 plasmid (under the ''lacI'' promoter). Initial and final conductance readings were taken. Continuous readings could not be taken since the team only had access to one conductivity probe at the time. Nevertheless, the control remained the same before and after lysis had occurred in the experimental. | ||
Revision as of 03:55, 30 October 2008
Optical DensityIn order to test the mechanism of the SRRz lysis cassette, we took optical density measurements as cell lysis occurred. The construct we used for testing was contained on the pVJ4 plasmid. This plasmid was obtained from the Mekalanos lab at HMS and contained the SRRz gene cassette in a pBAD18 plasmid. Initially we introduced arabinose to the culture of cells, allowed the cultures to sit at room temperature for several hours and measured the optical density before and after the cells lysed. The results are displayed in Figure 1. It was observed that when cells lysis occurred, the solution of lysing cells cleared after a few hours, providing qualitative evidence that lysis occurred (Figure 2).
Next, we wanted to test the amount of time required for lysis to occur. We added 0.2% by volume of an arabinose stock solution to cell cultures and measured the optical densities of the cultures at discrete time points. The following graphs exhibit optical density trends during gene expression and resulting cell lysis and cell wall degradation. Figure 3A shows a test measuring optical density and correlates that data to a predicted change in resistance that should occur as the cells lyse. Figure 3B shows another test of change in optical density over time.
Optical density of the cell cultures significantly dropped between 1 and 2 hours after induction, signifying the expression of the lysis gene cassette. Notice that optical density increases slightly within the first hour of the test, due to continued cell growth before the lytic event. The predicted change in resistance reflects this period of cell growth.
NaCl Resistance Testing
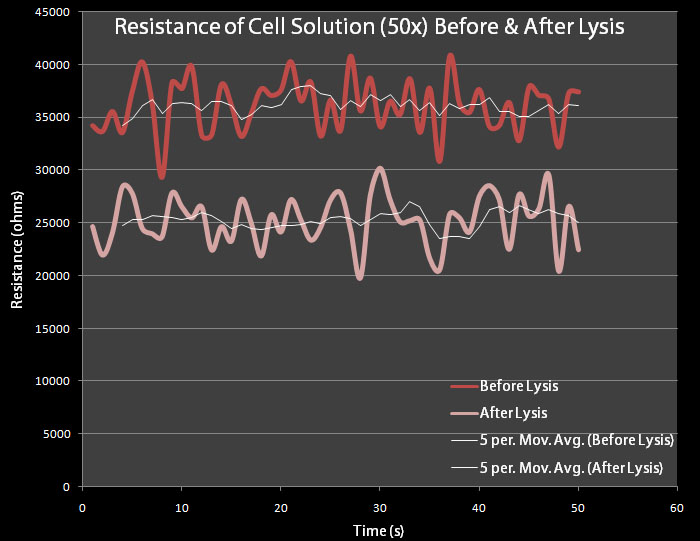
Bacterial Resistance TestingIn order to test whether a change in resistance could be detected by a 50x concentration of cells, a test was conducted in which the resistance of the supernatant of a 50x concentrated cell solution was measured before and after lysis.
500 mL dilutions of both pRG1 and pVJ4 were made in LB Lennox and then centrifuged. The giant pellets were resuspended in 10mL of M9 Minimal Media. 0.2% arabinose was added to the pVJ4 culture with a 50% Arabinose Solution Our resistance apparatus recorded measurements overnight. Figure 6 shows the resistance readings before lysis and after lysis. As expected there is a decrease in resistance.
Conductivity Testing
|
 "
"