Team:Cambridge/Signalling/Lab Work
From 2008.igem.org
Tasslehoff (Talk | contribs) |
|||
| Line 1: | Line 1: | ||
| + | {{Cambridge/Notitle}} | ||
| + | <div class=bodytable> | ||
| + | {{Cambridge08}} | ||
| + | {{Cambridge08signalling}} | ||
| + | <html> | ||
| + | <table style="background:#444444; padding:15px;"> | ||
| + | <table align=left width= border="0" style="background:#444444; padding:5px;"> | ||
| + | <tr> | ||
| + | <td style="width:100%; height: 400; padding-left: 15px;"> | ||
| + | <b class="b1f"></b><b class="b2f"></b><b class="b3f"></b><b class="b4f"></b> | ||
| + | <div class="contentf"> | ||
| + | <div style="height: 400; background: white; line-height:170% padding: 5px;"> | ||
| + | <div style="color: white; font: 2px;">x</div> | ||
| + | </html> | ||
| + | |||
=July, 22nd= | =July, 22nd= | ||
| Line 1,356: | Line 1,371: | ||
| - | < | + | <html> |
| - | + | <div style="color: white; font: 2px;">x</div> | |
| - | + | </div></div> | |
| + | <b class="b4f"></b><b class="b3f"></b><b class="b2f"></b><b class="b1f"></b> | ||
| + | </td></tr></table></table> | ||
| + | </html> | ||
| - | + | __NOEDITSECTION__ | |
Latest revision as of 04:15, 30 October 2008
|
x
July, 22ndDry work
July, 24thSingle Colony
Incubate overnight at 37°C July, 25thResults from Yesterday
Results Nothing! 1A1 cells were kept in the fridge! B.S. can not be kept in the fridge, low temperatures kill them!
July, 28thResults from previous days
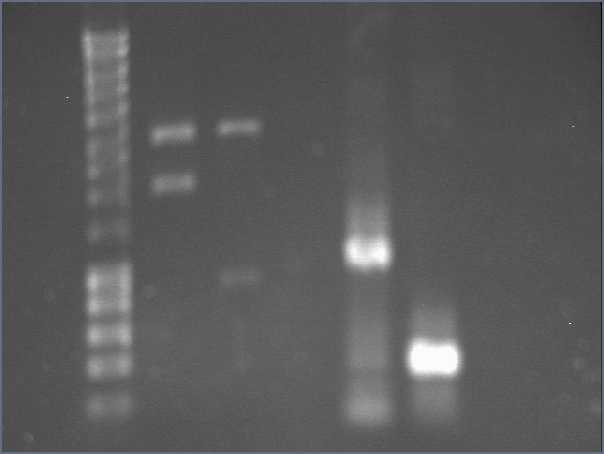
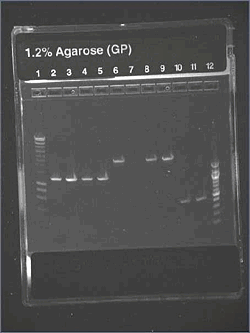
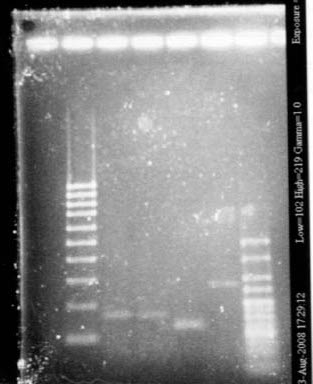
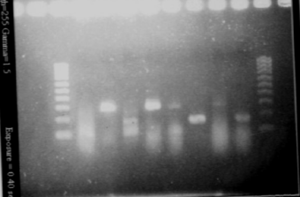
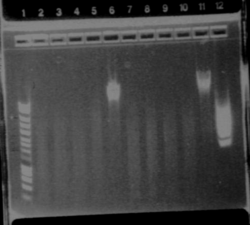
We wanted to check the size of our biobricks. For I746101, we have a band of about 3000kb, which is the size of the vectorm and a band of about 2000kb. The size of the biobrick is 2057kp, so it should be ok. For I746001, we hqve the same band of 3000kb (the vector) and a band of about 1000kb. The size of the biobrick is 896kb. So, the size of our biobricks are ok.
Wet Work- Digest
- Gel : add 4μL of dye and 21μL of samples
-Plate I746001 and I746101 on Kn plates from Amp plates from 24/07/2008. July, 29thResult from yesterday
The ladder seems to be wrong. So it was really difficult to ckeck the size of our plasmid ppL82. To make, that, we assume that the size of our biobricks was ok, and we estimate the size of our plasmid. The plasmid ppL82 seems to have the right size, but we will have to check again to be able to use a ladder. July, 30thWet Work
- Plamsid miniprep (for I746001, I746101 and PNZ8901 from big flasks - Measure DNA concentration in our samples to decide the volume of DNA we have to add in our preparation
Everthing is really too big! There is a problem, either dimerization, either contamination, either a problem in our work. So we are going to run a new gel.
- Miniprep plasmid from growth bottles (I746001, I746101 and PNZ8901); from plates (I746001, I746101 and PNZ8901). - Do single digest for PNZ8901 (one with PstI, one with SalI) - Run a gel with : PNZ8901 from friday, PNZ8901 digest with PstI, PNZ8901 digest with SalI, 3 samples from growth bottles, 3 samples from plates (to check the size of the uncut vectors)
The ladders are really bad! But the size of our biobricks and plasmid are too big! So we can not trust these big cultures. They is a problem. With tests from the first week, we know that biobricks are right, so we are going to grow new cultures from these first cultures, to make stocks. Concerning the vectors, they are from last year, so we are not sure of what they are. Since we ordered new well defined vectors (we should receive them on friday), we will use them in the next steps to be sure of our work. July, 31stWet workWe want to make stocks of biobricks I746001 and I746101, since our big cultures have a problem, we want to make some stocks from our first plates (which are good). We used original Amp plates from 23/07/08 of I746001 and I7461001. We took 5 single colonies and spotted on new Amp plates and inoculated in 10mL of LB with Amp. August, 1stWet Work
We had 5 single colonies for each biobrick fro; yestrday. We are going to PCR each single colony. Add 1μL of cells, 7 μL of SDW, 10μL of Phusion Flash master mix, 1μL of VF2 primer and 1μL of VR primer.
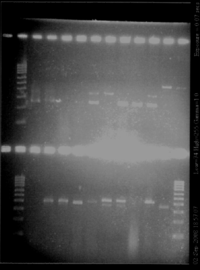
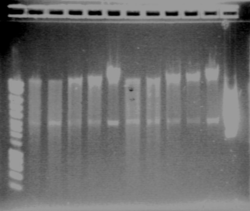
The result is good. Each colony of I746001 is about 1000kb (all the same size), for I746101, the size is good (about 2000kb), except for one single colony for which there is nothing. It could be a problem during the manipulation.
We have colony1 for each biobrick. We want to check them. - Plasmid miniprep - Digest with EcoRI and SpeI - Run a gel (for cut plasmid, add 19μL of DNA and 1μL of dye; for uncut plasmids, add 2μL of DNA, 17μL of SDW and 1μL of dye; for PCR product, add 5μL of DNA, 14μL of SDW and 1μL of dye)
- Result : everything is ok except for biobrick I746001, we have only one band - New double digest for biobrick I746001 (lane9), new gel : ok! We are sure to have some good biobricks for agr (I746001 and I746101) August, 5thTesting AHL degradation by aiiA
We are testing B. subtilis strain 168’s ability to degrade AHL by exposing synthetic AHL to the strain and then testing its ability to be recognized by the receiver.
Bacillus subtilis - protease deficient (NPR and APR) derived from strain 168 - grown up in an over night culture of LB. Synthetic AHL – Sigma K3007 – AHL powder was dissolved in EA buffer and diluted to 10uM concentration E. coli with T9002 AHL receiver grown up in overnight culture of LB All plates contain soft agar with 200uL of E. coli AHL receiver mixed-in. Plates 1,2 and 4 contain Bacillus in fresh LB medium, while plate 5 contains Bacillus in it’s overnight LB. Plate 5 was used to test if an excreted product of Bacillus is responsible for the degradation of AHL. The product of the aiiA gene in Bacillus subtilis is most likely not exported.
plates visualized 3 hours after AHL/Bacillus inoculation.....
August, 6thPCR parts of Agr operonWe received primers for Agr A,B,C, and D today - http://openwetware.org/wiki/IGEM:Cambridge/2008/Turing_Pattern_Formation/Primers We must PCR each individual part out of the previous BioBricks (I746001 &I746101) in order to put our bacillus RBS on them. I was concerned that the region of most homology would be the BioBrick prefix and suffix itself, so I first digested the DNA with Xbal and SpeI. For some reason the digestion didn't work that well, so I've decided to go ahead and use the uncut BioBrick DNA as template for my reactions. August, 9thPlans for MondayLab Work
Book/Computer Work
Longer Term Plans
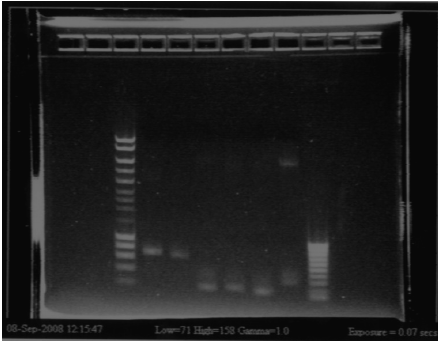
August, 11thPCR Bacillus RBSWe created 2 different Bacillus RBS sites simply by primer annealing and extension. Each product includes an 8bp RBS plus the BioBrick prefix and suffix for a total of 54bp. The products were visualized on a 3.5% Agarose gel with bioline's hyperladder V. RBSs is the consensus RBS sequence for Bacillus subtilis, and thus we believe it to be a very strong binding site - AAAGGAGG RBSw has a 2bp modification from RBSs, which we believe will weaken it - AGAGGTGG
RBSw, however produced 2 bands on the gel. The correct size was gel-extracted and purified. After purification the yield was 12.3 ng/uL. August, 13thPCR Agr A, B, C, DDoing a PCR directly from the old Biobricks has worked for all parts of the operon. No prior digestion seems to be needed.
August, 15thPCRing out promoters- 4 different promoters : Pxyl, Pspc, Ppac, Pupp - Make master mix enough for 4RXNs
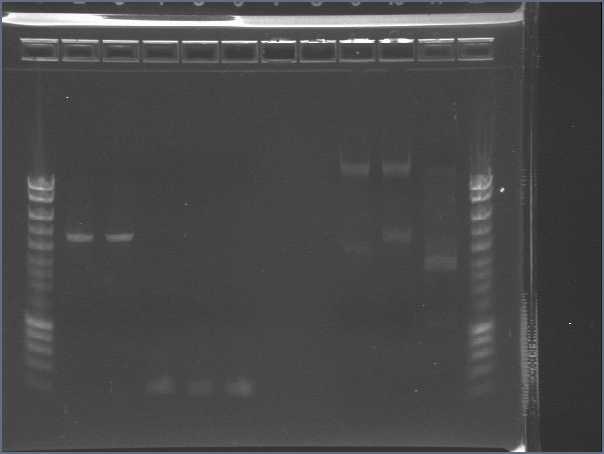
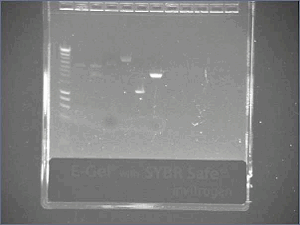
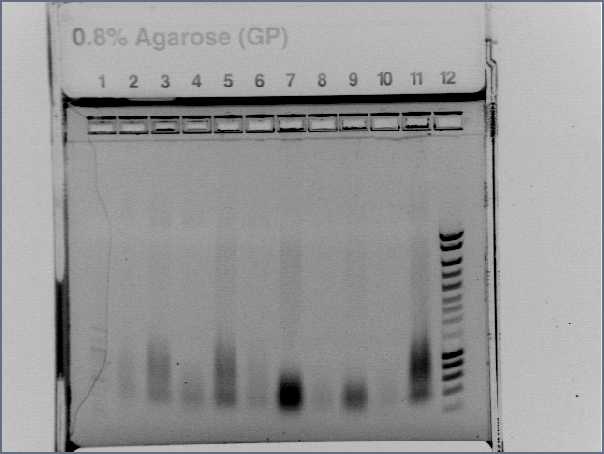
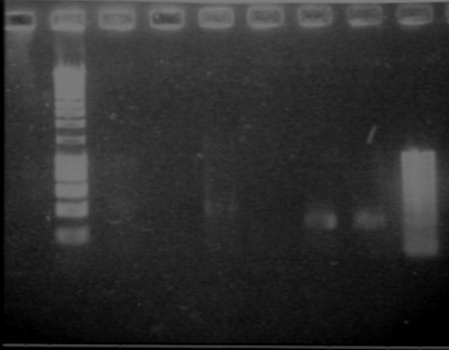
Digest and Ligate Agr A and C to pSB4C5Previous PCR products of Agr A and C were digested using Fermentas Fast Digest - EcoRI and PstI according to their protocol except a longer time was given to digest. 3uL of AgrA was used and 4.4 uL of AgrC was used and incubated for 40minutes. 10uL totaling to 1ug of pSB4C5 plasmid was also digested and incubated for 10 minutes. The digestion was visualized on a 0.8% Agarose gel and produced expected sizes: Plasmid: approx 3bk Agr A: approx 700bp Agr C: approx 1300bp
August, 16thAgr A and C ligation to pSB4C5Ligation was performed using Fermentas Rapid Ligation kit according to their protocol. After ligation, samples were visualized on a gel, but little product of the correct size was seen. For agrA we should have gotten a band of size 3700 (3000plasmid + 700 insert) For agrC we should have gotten a band of size 4300 (3000plasmid + 1300insert) The bands of appropriate sizes were extracted for transformation. August, 18thCheck promotersOn Friday, PCR out promoters, we want to check them. - Run a gel - Results : good size of bands!!! Transformation of AgrA and AgrCAgrA and C gell-extracted ligation was transformed into Top 10 cells using standard protocol. Puc9 was used as a positive control. August, 19thResults of AgrA and C transformationTransformation has failed. No colonies were visible for the plates of Agr A or C. The Puc9 positive control grew. We believe that the problem was in the gel-extraction between ligation and transformation. Most of our plasmid was probably lost in this step. Next time we will directly use the results from the ligation reaction to transform. Although many bands were seen on the previous gel of the ligation reaction, we will check our transformation growth by single colony PCR to confirm transformation of the plasmid with our correct insert. Lux partsTo make the Lux Receiver, we need 4 different parts ;
All these parts have been transformed into E.coli. We want to test them. R004, R0062 and JO4630 have already been tested, it should be fine. We received from the MIT R0040, R0062 and S068 already transformed into E.coli, so we want to check these stocks (which are certainly fine) and use them. For JO4630, we want to double check our transformation. - Plate on antibiotic plates and do LB stocks of single colony from the MIT stock (R0040, R0062 and S0168). - Put on Kan plates 4 different colonies from J04630 (transformation Amp plate) and also incubate these colonies into LB August, 20thCheck Lux components- Single colony PCR for :
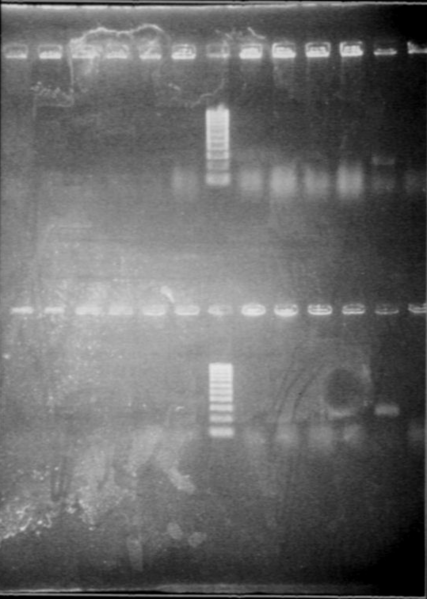
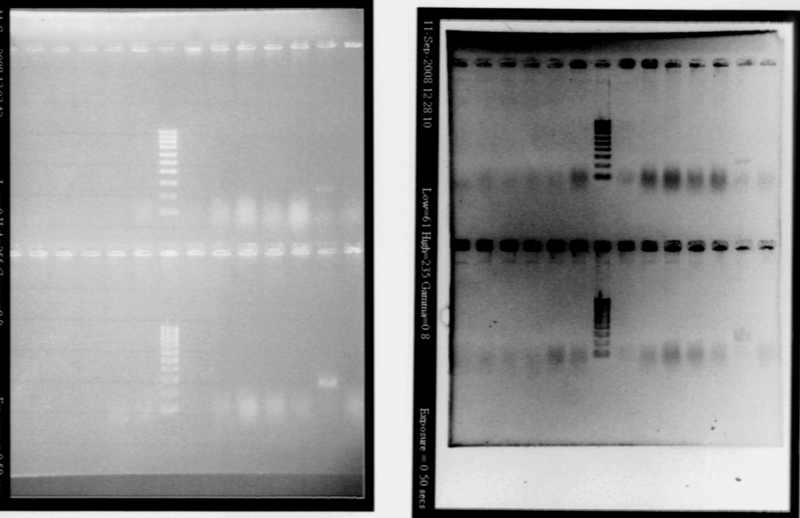
- Protocol : add 1μL of cells (diluted in water), 10μL of Master Mis, 7μL of SDW, 1μL of VF primer and 1μL of VR primer - Gel PCR products Gel 1
Gel 2
- Results
Ligation- Materials :
- Double digest of PCR products - Run vector, AgrA and AgrD on a gel - DNA clean and concentrator for AgrA, B,C and D, promoters - Microclean for both RBS - Nanodrop
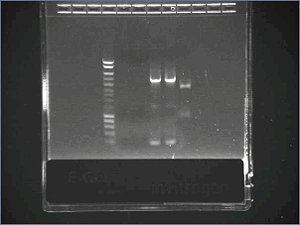
- Extract plasmid annd Agr from gel and clean - Ligation August, 21stTransformation of ligation products- Spin chemically competent TOP10, add 100μL of CaCl2 solution - Add 5μL of DNA (ligation products), and 1μL of PUC9 - Continue the protocol of transformation J04630- Plate good colonies from yesterday on Kan25 and put in LB (for plasmid stock for tomorrow) Plasmid stocks- Plasmid miniprep R0040 and R0062 August, 22ndNew PCR of promotersAugust, 23rdCheck promoters (after PCR from bacillus vectors)- Gel
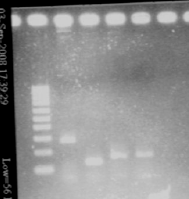
Size is ok. August, 27thNew transformation of ligation products
- Competent top 10 from the freezer - Add 5μL of ligation products, and 1.2μL of PUC9 August, 28thResults from transformation of ligation products- nothing on plate, even on control plate
- cells non competent anymore (try with fresh competent cells) - not enough DNA (try with 10μL of DNA) - very short ligation products Transformation of ligation products (new)
- new fresh competent TOP 10 - 5μL of DNA (1.5μL of PUC9) - 2h30 in the incubator August, 29thResults of transformation with our ligation products
Single colony PCR to check our transformation
- Single colony PCR : 13μL of SDW+cells, 5μL of Master Mix, 1μL of VF and 1μL of VR (and plate each single colony) - Load a gel (1.3% agarose) : 5μL of PCR products + 1μL of dye (only 1μL of 100b ladder) In the death plasmid, the VF-VR size is about 280b.
- run again on a e-gel
Plate biobricks from MIT
September, 2ndCheck transformation in E.coli of our ligated products- Single colony PCR with VF and VR : add 10μL of SDW, 5μL of MM, 1μL of VF, 1μL of VR and 3μL of cells
- Gel (3% of agarose)
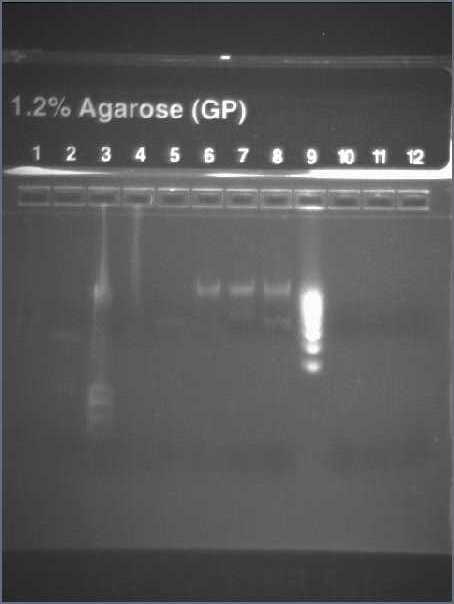
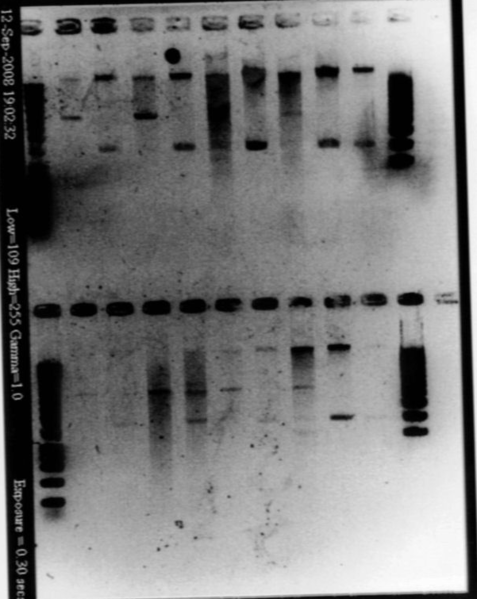
September, 3rdChecking the insert of promotersWe ligated promoters into death vector, and transforned into Top 10. Our transformation worked, but it was hard to say with the result of yesterday if we had the good insert. Moreover, it is possible that we inverted Pupp and Pspac. So we are going to make a new single colony PCR with the primers of promoters (we used the same single colonies than yesterday). We also want to PCR again all promoters (Pspac, Pxyl, Pupp and Ppac) from plasmids to have more. Picture of the gel with the different promoters
- Single colony PCR : add 10μL of SDW, 5μL of MM, 1μL + 1μL of primers, 3μL of cells - Gel
- Results
Make some stock of our new biobricks
- Grow this single colony into 10mL of LB (Cm35)
Transformation of new "biobricks"- transform into E.coli :
September, 4thResults of new "biobricks"transformation- Nothing on our plate, except for the control plate. New attempt of new "biobricks" transformation- transform into E.coli these ligated products:
September, 7thNew PCR- New stocks of Master Mix - PCR agrA, agrB, Pxyl, Pspac, Ppac and Pupp September, 8thCheck product of PCR from yesterday
Check transformation from 03/09After several days of incubation, we have a few colonies on our transformed plates (agrA, B and C).
- Add 13μL of cells diluted into SDW, 5μL of MM, 1μL of VF and 1μL of VR
September, 10thPCR of GFP+RBS and Promoter+RBS- PCR - Run a 1.2% agarose gel with 1μL of sample
RBS screening
- PCR : 11μL of SDW + 5μL of MM + 1+1μL of primers (RBS detect + VR) + 2μL of cells (program iGEM34) - Gel1
- Gel2
- Nothing, except for the PCR with VF and VR primers, either our primers are not right, either we have no insert. But the gel is not good enough to see very well, we will try to run a new gel. September, 11thRBS screening- We run our PCR products from yesterday on a new 1.8% agarose gel - Gel1
- Gel2
- Nothing, except for the PCR with VF and VR primers, either our primers are not right, either we have no insert. RBS screning but single digest- Grow some colonies of Top10 transformed with RBS into 10mL of LB with antibiotic We want to check our transformation with single digest. If we have self ligation in our transformation, we will loose the XbaI cutting site. Backbone for ligation- New pellets in 600μL of SDW - Plasmid miniprep September, 12thRBS screening (single digest)- Plasmid miniprep 6 colonies of RBS S and 6 colonies of RBS W (without endo wash buffer) - Nanodrop
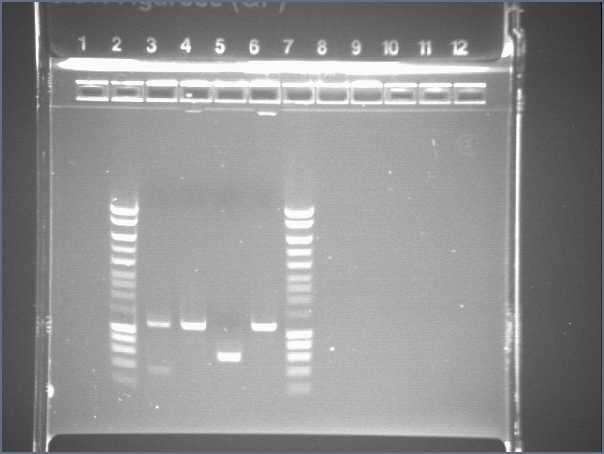
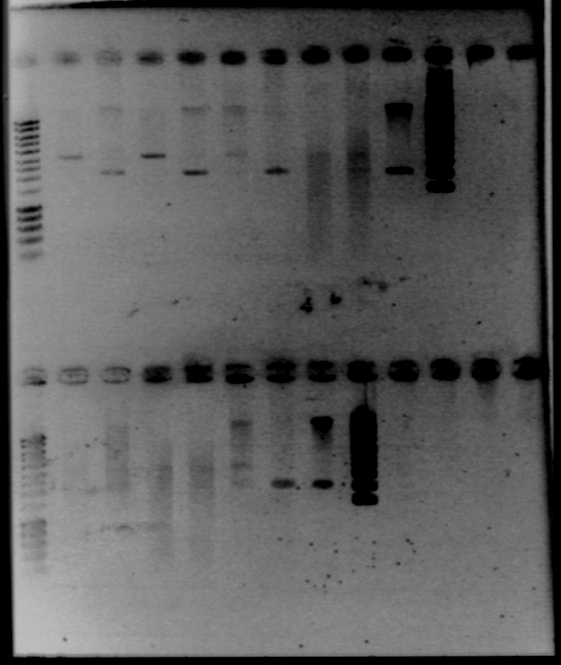
- Single digest : with EcoRI and XbaI, add SDW and DNA (according to the previous table, 300mg of DNA), 2μL of fast digest buffer, and 1μL of enzyme - Gel1
The size of our plasmid is about 3000bp (ok on the gel)
- W1 with EcoRI : cut - W1 with XbaI : uncut, self ligation - W3 with EcoRI : cut - W3 with XbaI : uncut, self ligation - W7 with EcoRI : impossible to see - W7 with XbaI : uncut, self ligation - W9 with EcoRI : cut - W9 with XbaI : uncut, self ligation
- S4 with EcoRI : cut - S4 with XbaI : uncut, self ligation - S6 with EcoRI : cut - S6 with XbaI : 2 band, this plasmid is partially cut!!! Transformation with RBS S - S8 with EcoRI : cut - S8 with XbaI : uncut, self ligation - S11 with EcoRI : cut - S11 with XbaI : uncut, self ligation Double check of RBS S6 and stock- Grow RBS S6 in LB with Cm35 Check more RBS W- Grow RBS W2, 4, 6, 8, 10 in 10mL of LB with antibiotic Check PCR products- Gel (2μL of each product)
- agr B : 2 bands? - agrC : ok - rep : small band but good size - backbones : ok - promoters : ok - RBS : nothing September, 13thGrow RBS S6- Put the 10mL of LB with RBS S6 from yesterday into 200mL of LB with antibiotic - Incubate at 37°C with shaking RBS W screening- Plasmid miniprep of RBS W2, 4, 6, 8, 10 9witouh endo wash buffer) - Nanodrop
- Single digest with EcoRI and XbaI - Gel1
- W2 with EcoRI : cut - W2 with XbaI : uncut, self ligation - W4 with EcoRI : cut - W4 with XbaI : uncut, self ligation - W5 with EcoRI : cut - W5 with XbaI : uncut, self ligation - W6 with EcoRI : cut - W6 with XbaI : cut (2 bands), transformation ok for RBS W6 - W8 with EcoRI : impossible to see - W8 with XbaI : impossible to see - W10 with EcoRI : cut - W10 with XbaI : maybe cut, it seems to be 2 bands, but quite difficult to see - W11 with EcoRI : cut - W11 with XbaI : uncut, self ligation Double check of RBS W6 and stock- Grow RBS W6 in 10mL of LB with antibiotic September, 14thRBS S6 and RBS W6- Put the 10mL of LB with OBS W6 in 200mL of LB with antibiotic - Aliquot the 200mL of LB with RBS S6, spin, throw out the supernatant and freeze the pellet September, 15thCheck big stock of RBS S and W
- Plasmid miniprep RBS S6 and RBS W6 (from 200mL flask) - Nanodrop
- Single digest with EcoRI, XbaI, SpeI and PstI - Gel
- Even no band for the uncut plasmid. There is a problem with plasmid miniprep, we will try again with less (more diluted) cells for plasmid miniprep. - New plasmid miniprep (the pellets from 20mL of LB is diluted into 1.2ml instead of 0.6) - New single digest - New gel (same lanes than before)
- One band for everything, but the same size than the uncut plasmid. Moreover, this uncut plasmid should be 3000bp, and here, it is more. So, we have a problem in the growth of cells, we will try again and check the 10ml tube which is used to inoculate the big flask and also the big flask.
x
|
 "
"