Team:BCCS-Bristol/Calendar-Notebook/15 July 2008
From 2008.igem.org
(Difference between revisions)
Rodgerread (Talk | contribs) |
Rodgerread (Talk | contribs) |
||
| Line 42: | Line 42: | ||
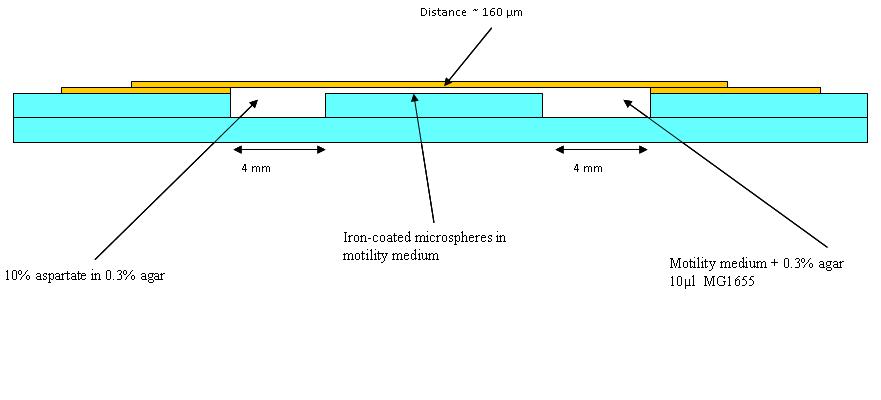
| - | had a go at making wells as in the diagram ( | + | had a go at making wells as in the diagram (See below), needs diff glue. then put a blue dye in 0.3% agar (to represent aspartate) and an orange dye in 0.1% agar to represent the bacteria. took pictures at 30minute intervals. this was to see how long a chemotatic gradient would take to set up, however after 3 hours no gradient was obious so left iovernight. |
| - | Recorded motility comparison, turns out we messed up and tried to grow MG1655 on streptomycin, therefore we made a repeat of this | + | Recorded motility comparison, turns out we messed up and tried to grow MG1655 on streptomycin, therefore we made a repeat of this experimen |
| + | |||
| + | [[Image:BCCS_Bristol-Wetlab-Well_diagram.JPG | 800px]] | ||
Revision as of 16:07, 24 July 2008
Made SOC medium in preparation for Biobrick transformation this consisted of
| Trypton | 2g |
| Yeast Extract | 0.5g |
| NaCl | 0.05g |
in 95ml of water, then autoclaved. after this add this:
| MgCl2 | 0.5ml 2M solution |
| Glucose | 2ml 1M |
then freeze at -20oC. this solution is from Molecular Cloning 3 A.2 SOB/SOC Medium.
had a go at making wells as in the diagram (See below), needs diff glue. then put a blue dye in 0.3% agar (to represent aspartate) and an orange dye in 0.1% agar to represent the bacteria. took pictures at 30minute intervals. this was to see how long a chemotatic gradient would take to set up, however after 3 hours no gradient was obious so left iovernight.
Recorded motility comparison, turns out we messed up and tried to grow MG1655 on streptomycin, therefore we made a repeat of this experimen
 "
"