Team:Chiba/Calendar-Home/31 August 2008
From 2008.igem.org
(Difference between revisions)
(→Team:Output) |
|||
| Line 155: | Line 155: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA </td> | + | <td>DNA(μL)</td> |
<td>10</td><td>10</td> | <td>10</td><td>10</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>SpeⅠ</td> | + | <td>SpeⅠ(μL)</td> |
<td>1</td><td>0</td> | <td>1</td><td>0</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PstⅠ</td> | + | <td>PstⅠ(μL)</td> |
<td>1</td><td>1</td> | <td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>XbaⅠ</td> | + | <td>XbaⅠ(μL)</td> |
<td>0</td><td>1</td> | <td>0</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>BSA(x100)</td> | + | <td>BSA(x100)(μL)</td> |
<td>0.5</td><td>0.5</td> | <td>0.5</td><td>0.5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Buffer2</td> | + | <td>Buffer2(μL)</td> |
<td>5</td><td>0</td> | <td>5</td><td>0</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>Buffer3</td> | + | <td>Buffer3(μL)</td> |
<td>0</td><td>5</td> | <td>0</td><td>5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>28.5</td><td>28.5</td> | <td>28.5</td><td>28.5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>50</td><td>50</td> | <td>50</td><td>50</td> | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | --> | + | -->left for 3 hours at 37 degrees. |
-->①20μl refine | -->①20μl refine | ||
-->②20μl refine | -->②20μl refine | ||
Revision as of 11:32, 24 October 2008
30 August 2008 <|> 1 September 2008
Contents |
Laboratory work
Team:Input
no work
Team:Communication
- Transformation
- Competent Cells : XL10G
- [http://partsregistry.org/Part:BBa_K084007 BBa_K084007(Plac+RBS+LasI)]
- [http://partsregistry.org/Part:BBa_K084008 BBa_K084008(Plac+RBS+RhlI)]
- ---> We saved these plates with a refrigerator.
- Digestion
- [http://partsregistry.org/Part:BBa_I9026 BBa_I9026](2007)
- [http://partsregistry.org/Part:BBa_I9030 BBa_I9030](2006)
- [http://partsregistry.org/Part:BBa_S03154 BBa_S03154](2007)
- [http://partsregistry.org/Part:BBa_R0010 BBa_R0010](2007)
Sample No. 1~3 4 Sample DNA 12 10 PstⅠ 0.2 0.2 XbaⅠ 0.2 - SpeⅠ - 0.4 Buffer 2 - 1.5 Buffer 3 2 - BSA 2 1.5 dH2O 3.6 1.4 TOTAL 20 15
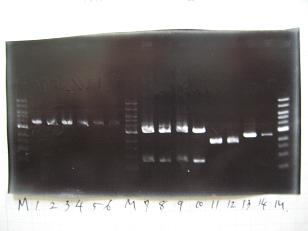
- --->(1/9)Gel Check
- (30/8)--->Mini prep
- --->Digestion test
- Plac+RBS+RhlI Sample No.2~5
- [http://partsregistry.org/Part:BBa_T9002 BBa_T9002]①、②
Sample Single Digesiton 2~5 Double Digestion 2~5 Single Digestion T9002① Single Digesiton T9002② Double Digestion T9002①② Sample DNA 1 3 3 1 3 XbaⅠ 0.1 0.1 0.1 0.1 0.1 SpeⅠ - 0.1 - - 0.1 Buffer 2 0.9 0.8 0.9 0.9 0.8 BSA 1 1 1 1 1 dH2O 7 5 5 7 5 TOTAL 10 10 10 10 10
- --->Gel Check
Team:Output
- [http://partsregistry.org/Part:BBa_R0010 BBa_R0010]①
- PCR product([http://partsregistry.org/Part:BBa_J52008 BBa_J52008])②
Sample No. ① ② DNA(μL) 10 10 SpeⅠ(μL) 1 0 PstⅠ(μL) 1 1 XbaⅠ(μL) 0 1 BSA(x100)(μL) 0.5 0.5 Buffer2(μL) 5 0 Buffer3(μL) 0 5 dH2O(μL) 28.5 28.5 TOTAL(μL) 50 50 -->left for 3 hours at 37 degrees.
-->①20μl refine
-->②20μl refine
 "
"