Team:Chiba/Calendar-Home/1 September 2008
From 2008.igem.org
(Difference between revisions)
(New page: >Home | Notebook 31 August 2008 <|> 2 September 2008 ==Lab...) |
(→Team:Input) |
||
| Line 16: | Line 16: | ||
<td>Double</td><td>Single</td> | <td>Double</td><td>Single</td> | ||
<tr> | <tr> | ||
| - | <td>dH<sub>2</sub>O</td> | + | <td>dH<sub>2</sub>O(μL)</td> |
<td>1</td><td>2</td> | <td>1</td><td>2</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>XbaI</td> | + | <td>XbaI(μL)</td> |
<td>1</td><td>1</td> | <td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>SpeI</td> | + | <td>SpeI(μL)</td> |
<td>1</td><td>-</td> | <td>1</td><td>-</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>BSA(×10)</td> | + | <td>BSA(×10)(μL)</td> |
<td>1</td><td>1</td> | <td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>NEB(×10)</td> | + | <td>NEB(×10)(μL)</td> |
<td>1</td><td>1</td> | <td>1</td><td>1</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>DNA</td> | + | <td>DNA(μL)</td> |
<td>5</td><td>5</td> | <td>5</td><td>5</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>TOTAL</td> | + | <td>TOTAL(μL)</td> |
<td>10</td><td>10</td> | <td>10</td><td>10</td> | ||
</tr> | </tr> | ||
| Line 46: | Line 46: | ||
| - | + | UV irradiation test | |
| - | *-Ptrc-LuxR-Plux-cI-colE1-Amp- | + | *two plasmids from ? |
| - | + | **-Ptrc-LuxR-Plux-cI-colE1-Amp- | |
| - | + | **-PcI-GFP-p15a-Cm-の2plasmid(BW) | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | #Incubated cultures(from Glycerol Stocks) with 2mL of LB-Ampicillin Medium for 12 hours at 37 degrees. | ||
| + | #UV⊕:5.21,UV⊖:5.38<--? | ||
| + | #moved the cultures to small plates and started UV-irradiation.(wavelength:254nm,distance from the UV lamp to the cultures were 7.5cm.UV⊖のものは暗所に置いておく。) | ||
| + | #covered the plates with polyethylene wrap. | ||
| + | #after irradiation(Sample:0min,10min,30min,1h,2h,4h,6h,8h)(Negative Control:0h,1h,4h,8h) | ||
| + | よく攪拌してから、20μl採取。 | ||
| + | #diluted each cultures with LB-ampicillin medium 10<sup>4</sup>-fold and 10<sup>5</sup>-fold (volume/volume). | ||
| + | #incoculated 20μl of the cultures and incubated for 12 hours at 37 degrees. | ||
| + | #counted the CFU(determined viable cell count). | ||
| - | + | ||
| + | |||
| + | Viable cell count | ||
<table width="200" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | <table width="200" border="4" cellpadding="0" cellspacing="0" bordercolor="#000000"> | ||
<td width="257"></td> | <td width="257"></td> | ||
| Line 94: | Line 100: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | |||
===Team:Communication=== | ===Team:Communication=== | ||
Revision as of 12:09, 24 October 2008
31 August 2008 <|> 2 September 2008
Contents |
Laboratory work
Team:Input
- Ptet+RBS+cIの、Mini Prep産物の濃度チェック。
- -Ptet-cI-pMB1-Amp-の機能チェック。
- -Ptet-cI-pMB1-Amp-,-PcI-GFP-p15A-Cm-をDouble Transformation(BWΔflic)
- 結果-->GFPが発現していた。-->-PcI-GFP-p15A-Cm-が機能していないのでは?
- ターミネーターをつける、pSB1A3にのせかえる。
- -Ptet-cI-pMB1-Amp-のDigestion Check
混ぜ表
| Double | Single | |
| dH2O(μL) | 1 | 2 |
| XbaI(μL) | 1 | 1 |
| SpeI(μL) | 1 | - |
| BSA(×10)(μL) | 1 | 1 |
| NEB(×10)(μL) | 1 | 1 |
| DNA(μL) | 5 | 5 |
| TOTAL(μL) | 10 | 10 |
UV irradiation test
- two plasmids from ?
- -Ptrc-LuxR-Plux-cI-colE1-Amp-
- -PcI-GFP-p15a-Cm-の2plasmid(BW)
- Incubated cultures(from Glycerol Stocks) with 2mL of LB-Ampicillin Medium for 12 hours at 37 degrees.
- UV⊕:5.21,UV⊖:5.38<--?
- moved the cultures to small plates and started UV-irradiation.(wavelength:254nm,distance from the UV lamp to the cultures were 7.5cm.UV⊖のものは暗所に置いておく。)
- covered the plates with polyethylene wrap.
- after irradiation(Sample:0min,10min,30min,1h,2h,4h,6h,8h)(Negative Control:0h,1h,4h,8h)
よく攪拌してから、20μl採取。
- diluted each cultures with LB-ampicillin medium 104-fold and 105-fold (volume/volume).
- incoculated 20μl of the cultures and incubated for 12 hours at 37 degrees.
- counted the CFU(determined viable cell count).
Viable cell count
| UV+ 104 | UV+ 105 | UV-104 | UV-105 | |
| 0min | 423 | 74 | 271 | 156 |
| 10min | 301 | 51 | - | - |
| 30min | 139 | 7 | - | - |
| 1h | 89 | 5 | 1040 | 54 |
| 2h | 5 | 1 | - | - |
| 4h | 2 | 0 | 254 | 50 |
| 6h | 0 | 0 | - | - |
| 8h | 1 | 0 | 1155 | 106 |
Team:Communication
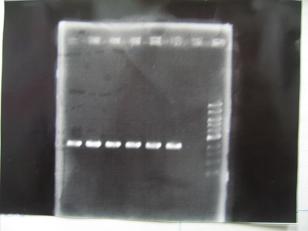
- (31/8)--->Gel Check

|
|
|
|
|
|
- --->Gel extract
- --->zymo
- insert-1(I9026) -> 7μL
- insert-2(I9030) -> 7μL
- insert-3(S03154) -> 7μL
- vector-4(R0010) -> 15μL
- --->SAP
- vector-4(R0010)
- --->Zymo
- vector-4(R0010) -> 20μL
- --->Gel Check

|
|
- --->Ligation
| Sample No. | (1) | (2) | (3) | (4) | (5) |
| insert-1(I9026) | 3 | - | 3 | - | - |
| insert-2(I9030) | - | 3 | - | 3 | - |
| vector-4(R0010) | 3 | 3 | - | - | 3 |
| ligase | 1 | 1 | 1 | 1 | 1 |
| Buffer | 1 | 1 | 1 | 1 | 1 |
| dH2O | 2 | 2 | 5 | 5 | 5 |
| TOTAL | 10 | 10 | 10 | 10 | 10 |
- --->Transformation
- Competent cells : XL10GOLD 30μL
- Transformed the following and grew on new ampicillin plates.
- [http://partsregistry.org/Part:BBa_K084009 BBa_K084009(Plac+RBS+RhlI+LVA, Amp)] -> 628 colonies
- [http://partsregistry.org/Part:BBa_K084010 BBa_K084010(Plac+RBS+CinI+LVA, Amp)] -> 500 colonies
- insert-1(RBS+RhlI+LVA) -> 9 colonies
- insert-2(RBS+CinI+LVA) -> No colonies on the plate
- vector-4(Plac, Amp) -> 186 colonies
- --->(2/9) Colony PCR
- Competent cells : JW1908 40μL
- Transformed the following and grew on new ampicillin plates.
- [http://partsregistry.org/Part:BBa_K084007 BBa_K084007(Plac+RBS+LasI)]
- [http://partsregistry.org/Part:BBa_K084008 BBa_K084008(Plac+RBS+RhlI)]
- [http://partsregistry.org/Part:BBa_K084010 BBa_T9002(Ptet+RBS+LuxR+GFP)]
- --->(2/9)Liquid Culture
Team:Output
- vector:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010] insert:[http://partsregistry.org/Part:BBa_J52008 BBa_J52008]①
- negative control:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010]②
| Sample No. | ① | ② |
| vector | 2 | 2 |
| insert | 3 | 0 |
| Ligase Buffer | 2 | 2 |
| Ligase | 1 | 1 |
| dH2O | 3 | 5 |
| TOTAL | 10 | 10 |
-->R/T 2hour
- [http://partsregistry.org/Part:BBa_R0079 BBa_R0079](Las promoter)
- [http://partsregistry.org/Part:BBa_R0071 BBa_R0071](RhlR promoter)
- [http://partsregistry.org/Part:BBa_R0077 BBa_R0077](cinR promoter+RBS)
- [http://partsregistry.org/Part:BBa_R0078 BBa_R0078](cinR promoter)
- [http://partsregistry.org/Part:BBa_R0062 BBa_R0062](Lux promoter)
 "
"