Team:University of Sheffield /Wet Lab
From 2008.igem.org
(→Wet Lab) |
|||
| Line 25: | Line 25: | ||
=Wet Lab= | =Wet Lab= | ||
| - | [[Image:general_plan.jpg| | + | [[Image:general_plan.jpg|600px|center|general plan]] |
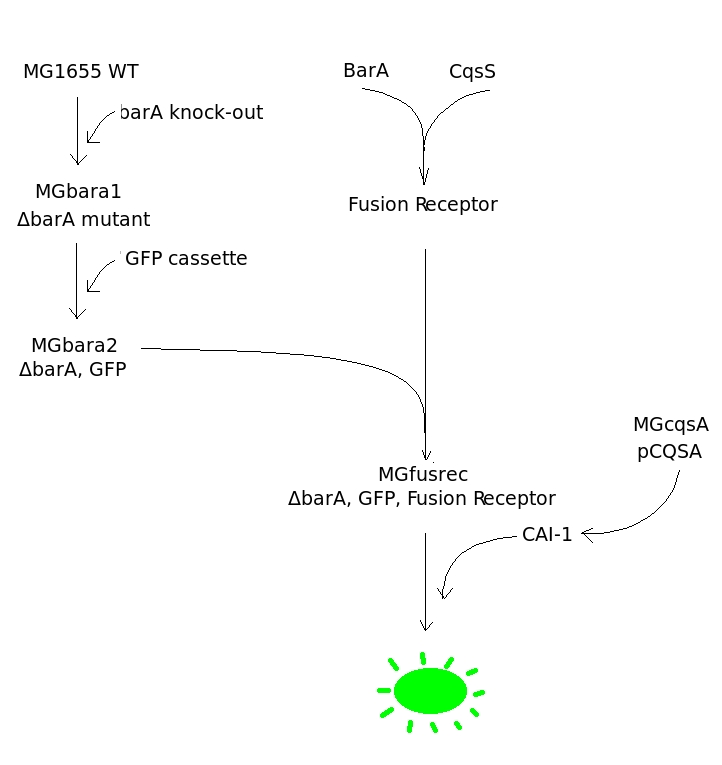
The Diagram is a stepwise representation of a plan we wanted to carry out to achieve our goal. First of all gene coding for BarA protein should be knocked out from wild strain of an engineered bacterium (E.coli MG1655). Then GFP should be introduced into ΔbarA mutant (MGbara1) under a promoter of a gene positively regulated by BarA. Simultaneously to those steps Fusion Kinase should be created and inserted into an expression plasmid. Then both intermediates, ΔbarA mutant with GFP in its genome (MGbara2) and plasmid with Fusion Kinase, should be combined together to give rise to the biosensor (MGfusrec). Functionality of a biosensor should be analyzed by exposing it to CAI-1 autoinducers. If the design is successful the bacterium should grow. | The Diagram is a stepwise representation of a plan we wanted to carry out to achieve our goal. First of all gene coding for BarA protein should be knocked out from wild strain of an engineered bacterium (E.coli MG1655). Then GFP should be introduced into ΔbarA mutant (MGbara1) under a promoter of a gene positively regulated by BarA. Simultaneously to those steps Fusion Kinase should be created and inserted into an expression plasmid. Then both intermediates, ΔbarA mutant with GFP in its genome (MGbara2) and plasmid with Fusion Kinase, should be combined together to give rise to the biosensor (MGfusrec). Functionality of a biosensor should be analyzed by exposing it to CAI-1 autoinducers. If the design is successful the bacterium should grow. | ||
Revision as of 09:37, 29 October 2008
| Introduction | Our project | Modelling | Wet Lab | Our team | Timetable | Miscellaneous |
|---|
| Introduction | Protocols |
|---|
Wet Lab
The Diagram is a stepwise representation of a plan we wanted to carry out to achieve our goal. First of all gene coding for BarA protein should be knocked out from wild strain of an engineered bacterium (E.coli MG1655). Then GFP should be introduced into ΔbarA mutant (MGbara1) under a promoter of a gene positively regulated by BarA. Simultaneously to those steps Fusion Kinase should be created and inserted into an expression plasmid. Then both intermediates, ΔbarA mutant with GFP in its genome (MGbara2) and plasmid with Fusion Kinase, should be combined together to give rise to the biosensor (MGfusrec). Functionality of a biosensor should be analyzed by exposing it to CAI-1 autoinducers. If the design is successful the bacterium should grow.
Click here, or on the navigation bar, for the overall timetable of our wet lab sessions.
Click here, or on the sub-navigation bar, for almost all the protocols we used in our wet lab sessions.
The lab books below contain the more detailed jobs carried out by each member of the team.
Lab Books
Plasmids and Primers
| Experimental Primers | ||||
|---|---|---|---|---|
| BarA homology
sequences on pKD13 Kanamycin resistant cassette | Forward:
5' - TGA TGA TTC TGA TCC TGG CAC CGA CCG TCC TTA TTG ATT CCG GGG ATC CGT CGA CC - 3' | TM (50mM NaCL): 71.8 °C | GC Content: 55.4% | Molecular Weight (Calculated): 17149.1 |
| Reverse:
5' - CGT TGA CTT CGG GCG TCA CGA CGC GAG AGG AAA TAC GTG TAG GCT GGA GCT GCT TC - 3' | TM (50mM NaCL): 72.6 °C | GC Content: 58.9% | Molecular Weight (Calculated): 17377.2 | |
| RFP insertion
into PGA operon = PGA homolgy on RFP cassette | Forward:
5' - TAA TTA TAC TCA CCA GCA TCA GGA GAT ATT TAT TTC CAT TAC GTA ACA TAT TTA TCC TTA TTA TTA AGC TAC TAA AGC GT - 3' | TM (50mM NaCL): 65.6 °C | GC Content: 28.8% | Molecular Weight (Calculated): 24492 |
| Reverse:
5' - AAC TGG CGC GGT TTT GCT GGA TTC GGT TAT GCC GAT GGA CAA TTT AGC GAA GGA AAA GGG ATG GCT TCC TCC GAA GAC GT - 3' | TM (50mM NaCL): 73.3 °C | GC Content: 51.2% | Molecular Weight (Calculated): 24870.1 | |
| Biobrick Primers | ||||
|---|---|---|---|---|
| BIOBRICK 1 + 2
CsrA with promoter +prefix | Forward:
5' - GAA TTC GCG GCC GCT TCT AGA ACA GAA TGT AAT GCC ATG AC - 3' | TM (50mM NaCL): 66.6 °C | GC Content: 48.8% | Molecular Weight (Calculated): 12618.2 |
| CsrA without
promoter +prefix | Forward:
5' - GAA TTC GCG GCC GCT TCT AGA TGC TGA TTC TGA CTC GTC GAG TTG - 3' | TM (50mM NaCL): 68.8 °C | GC Content: 53.3% | Molecular Weight (Calculated): 13850 |
| Reverse for both 1 & 2:
5' - CTG CAG CGG CCG CTA CTA GTA GTA ACT GGA CTG CTG GGA TTT TT - 3' | TM (50mM NaCL): 69 °C | GC Content: 52.3% | Molecular Weight (Calculated): 13569.8 | |
| BIOBRICK 3 + 4
CrsB with promoter +prefix | Forward:
5' - GAA TTC GCG GCC GCT TCT AGG TCG ACA GGG AGT CAG ACA AC - 3' | TM (50mM NaCL): 69.9 °C | GC Content: 58.5% | Molecular Weight (Calculated): 12660.2 |
| CsrB without
promoter +prefix | Forward:
5' - GAA TTC GCG GCC GCT TCT AGA GGG AGT CAG ACA ACG AAG T - 3' | TM (50mM NaCL): 69 °C | GC Content: 55% | Molecular Weight (Calculated): 12395.1 |
| Reverse for both 3 & 4
+suffix: 5' - CTG CAG CGG CCG CTA CTA GTA AAT AAA AAA AGG GAG CAC TG - 3' | TM (50mM NaCL): 66.5 °C | GC Content: 48.8% | Molecular Weight (Calculated): 12685.3 | |
| BIOBRICKS 5 & 6
UvrY with promoter +prefix | Forward:
5' - GAA TTC GCG GCC GCT TCT AGA ATG ACT AAC TAT CAG TAG CGT TAT C - 3' | TM (50mM NaCL): 65.5 °C | GC Content: 45.7% | Molecular Weight (Calculated): 14124.2 |
| UvrY without
promoter +prefix | Forward:
5' - GAA TTC GCG GCC GCT TCT AGT ATT CCT TTG ATC AAC GTT CTA C - 3' | TM (50mM NaCL): 65.7 °C | GC Content: 46.5% | Molecular Weight (Calculated): 13110.5 |
| Reverse for both 5 & 6
+suffix 5' - CTG CAG CGG CCG CTA CTA GTA TCA CTG ACT TGA TAA TGT CT - 3' | TM (50mM NaCL): 66.3 °C | GC Content: 48.8% | Molecular Weight (Calculated): 12551.2 | |
Plasmids:
- pKD46
- pKD13
- pCP20
- pCQSA
- pCQSS
 "
"