Team:Peking University/Project
From 2008.igem.org
(→Project Abstract) |
(→Project Abstract) |
||
| Line 26: | Line 26: | ||
{|align="center" | {|align="center" | ||
| | | | ||
| - | |[[Image: | + | |[[Image:New_General_Circuit.jpg|500px]] |
|} | |} | ||
</p> | </p> | ||
Revision as of 10:37, 29 October 2008

| Home | The Team | The Project | Parts Submitted to the Registry | Modeling | Misc&Fun | [http://www.openwetware.org/wiki/IGEM:Peking_University/2008 Our OWW] | [http://www.openwetware.org/wiki/IGEM:Peking_University/2008/Notebook Notebook] |
|---|
Contents |
Project Abstract
A Genetic Circuit for Directed Evolution in vivo
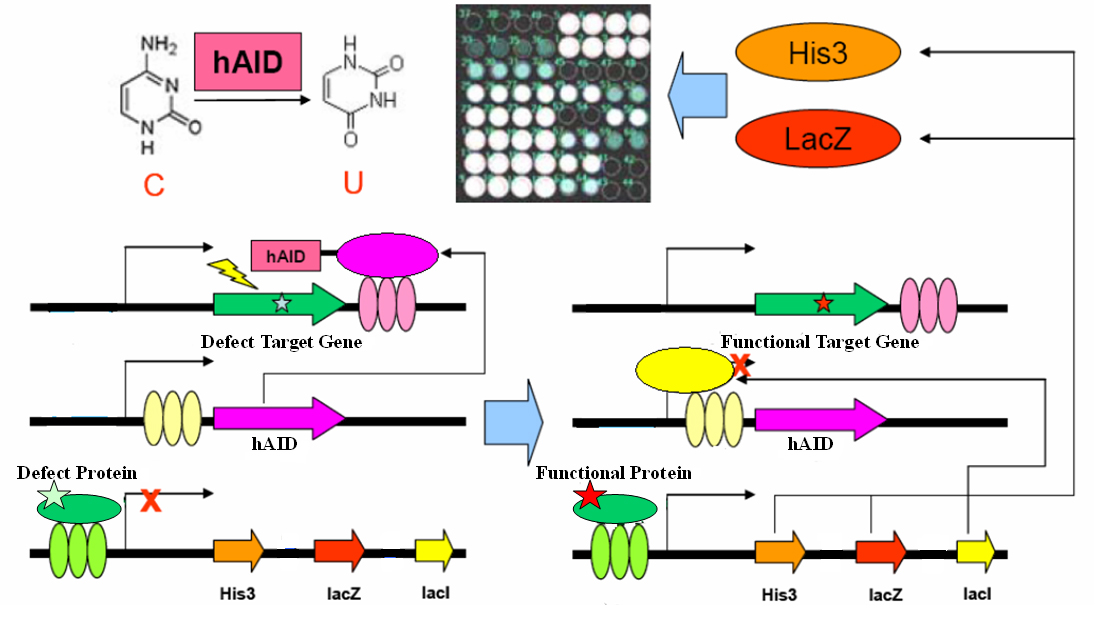
Directed evolution is a powerful tool for answering scientific questions or constructing novel biological systems. Here we present a simple genetic circuit for in vivo directed evolution which comprises minimal elements for random mutation and artificial selection. We engineer yeast to generate the DNA mutator hAID, an essential protein in adaptive immunity, and target it specifically to a gene of interest. The target gene will be mutated and consequently promptly evolves. By linking the expression of hAID repressor LacI and favorite gene functionality, the mutation rate inversely correlates between the functionality of the desired gene and hAID. This circuit may be adopted for in vivo evolution in eukaryotic system on genetically encoded targets. It has a variety of potential applications in academic and industrial contexts, theoretically most inter-molecular interaction that involves proteins and RNAs.
Genetic Circuit
|
Project Details
Project Proposal
Evolution could be dissociated into two parallel processes: random mutation, and natural selection. Whilst its role in life history is well understood and accepted, evolution is also evident across individual life process. In particular, adaptive immunity system adopts evolution strategy to produce antibodies for novel antigens. Artificial evolution method could be a powerful tool for answering scientific questions or engineering novel biological systems. Via systems biology approach, here we present a simple genetic circuit consisting functional elements for random mutation and artificial selection. This circuit may perform in vivo evolution on virtually any genetically encoded targets, with potential applications in academic and industrial contexts.
The gene encoding the core element in adaptive immunity, hAID, is fused with the LexA-DBD domain with flexible linker. The fusion gene hAID-LexA is inserted into a yeast ESC expression cassette at 3’ of tandem LacO elements. The target gene (your favorite gene, yfg) is inserted into a yeast ESC expression cassette, with its 3’UTR containing tandem LexO elements. An inducible promoter response to yfg activity drives expression of His3, LacZ and LacI in cistrone spanned by IRES. These three plasmids together form a genetic circuit for in vivo evolution.
For clearness, here we present one very simple example in yeast one hybrid: a mutated Gal4 gene is inserted in the target cassette. The selector cassette contains UAS to drive expression of His3, LacZ and LacI. All three plasmids are transfected or knock-in-ed into gal4-, his3- yeast strain with proper selection tags. Initially, we culture the yeast in complete YPD medium. Defect Gal4 product cannot bind to UAS, hence hAID-LexA is constitutively expressed, recruited to LexO sites of the target, and mutates the Gal4 conding sequence. Once Gal4 mutation is reversed, it binds to UAS to drive LacI expression, which represses hAID-LexA. We plate the yeast into Trp-, His-, Leu-, 3AT+, XGal+ plate and select for the large, blue colonies. Sequencing the colonies then gives us the activated Gal4 gene sequences.
The power of this system could be best revealed in the above case: since selection is not required for initiation and attenuation of mutagenesis, there is virtually no need to perform selective pressure titration.
The system could be readily adopted to in vivo evolution of any kind of protein-protein and protein-nucleic acid interaction: for this we simply adopt a yeast two hybrid-like approach, evolving a functional protein linked to Gal4 AD which could bind to the "bait" protein linked to Gal4 DBD. We could also use the yeast three hybrid system to study RNA-protein interaction, or use yeast one hybrid to evolve specific protein that binds to given promotor.
Yeast shows several advantages in studying eukaryotic process, for it folds and modifies the eukaryotic protein accurately, whilst bacteria does not. Another advantage of yeast is that cell wall blocks intracellular communication between transmembrane proteins, and the selection happens completely within the cell, therefore blocking possible false positives. We plan to screen for extracellular activator of certain eukaryotic transmembrane protein, for example, novel peptidergic ligand for GPCR and antibody-antigen interaction.
We have been working on further improvements of the mutation system, by improving the enzyme to archieve directed, "hotspot-less", and evenly distributed mutagenesis, by utilizing the overwhelming power of yeast genetics, and by harnessing novel protein chemistry.
//Last modified: ZY 20080704
Project Prospect
The figure plan consists 4 figures and 2 sets of equations in total:
Figure 1: a genetic circuit can conduct artificial evolution in yeast
- panel A, a schematic figure of molecular cloning of plasmids hAID and Gal4
- panel B, yeast with plasmid hAID and defect Gal4 can evolve blue colonies just as the yeasts with hAID and wild type Gal4 on non-selective medium, in contrast, yeast with only defect Gal4, or only hAID, cannot develop blue patterns.
- panel C, a quantitative time-series plating showing the evolution time course, and calculating phenotypic convergence rate of evolution
- panel D, sequencing results of the positive (blue) colonies, showing preference and mutation rate of this circuit
- panel E, sequencing results showing convergent of evolution on DNA sequence for phenotypical selection
- panel F, knock-in of evolved Gal4 can replace the wild type Gal4 on genome
Figure 2: a genetic circuit with hAID, Gal4 and LacI can confer directed artificial evolution in yeast
- panel A, a schematic figure of molecular cloning for plasmids hAID, Gal4, and LacI
- panel B, western blot with yeast with hAID, wild type or defect Gal4, and LacI, showing that hAID is only expressed in "defect Gal4" yeast, and LacI is only expressed in "wild type Gal4" yeast.
- panel C, quantitative time-series plating showing that this 3-component circuit is more probable to converge positive hits than the two-component circuit
- panel D, selective medium plating showing that the 3-component circuit is able to evolve under stringent selective pressure
Equation set 1: a probability model for hAID-directed evolution in vivo
Equation set 2: saturated screen and minimal start set of DNA sequence
Figure 3: the genetic circuit is applicable to negative selection
- panel A: principle of negative selection based on LacZ
- panel B: the genetic circuit consisting knockins hAID-LexA, Gal4-LexO, UAS-lacZ
- anel C: quantitative time-series plating showing negative selection based on blue-white screening
- panel D: the genetic circuit consisting knockins hAID-LexA, Gal4-LexO, UAS-LacI, and LacO-His3
- panel E: quantitative time-series plating showing negative selection based on nutrient deficiency
Figure 4: the genetic circuit is applicable to GPCR and Y2H screens
- panel A, construction of GPCR and Y2H circuits with hAID-LexA, reporter sequence-LacI, and mutation target-LexO
- panel B, in Y2H, we can evolve a proteins to interact with a specific target
- panel C, cell line co-IP experiments showing that the evolved protein interact with the specific target in vitro
- panel D, in GPCR screen, we can evolve a set of peptides that either activate or inactivate a specific GPCR
- panel E, ex vivo pharmacology showing that the positive hit peptides activate/inactivate the specific GPCR
We may have 2-3 supplementary figures for additional screens and quantitative information. I expect this work quite interesting.
 "
"