Running an MFC Experiment
This page is intended as a comprehensive guide to completing a microbial fuel cell setup and running an experiment from start to finish.
Creating a Testing Environment
Begin 1-2 weeks prior to experiment
Constructing Fuel Cell Components
Materials (per fuel cell)
- 4" Polycarbonate Square Tube, 2" Outer Diameter
- 6" x 6" Polycarbonate Sheet, 1/4" Thick
- 4 Steel Fully Threaded Stud, 1/4"-20 Thread, 6" Length
- 8 Zinc Alloy Wing Flange Nut, 1/4"-20 Screw Size, 1" Wing Spread
- 1" x 1" Nafion® membrane, 0.180mm thick
- 1" x 1" Carbon felt, 0.25" thick
- 1.5" x 1.5" E-TEK ELAT™ GDE (platinum on carbon)
- 2' Titanium Grade 2 Wire .046" Diameter
- Teflon Tape, 1/4" Width
- 5" x 2.5" Silicone Sheet
- Silicone Glue
- Spiral Point Tap 1/4"-28
- 8 Plastic Luer Lock Coupling Nylon, Female to Male Thread, 1/4"-28
- 8 Luer Lock Injection Ports
Procedure
- 1) Mill Polycarbonate
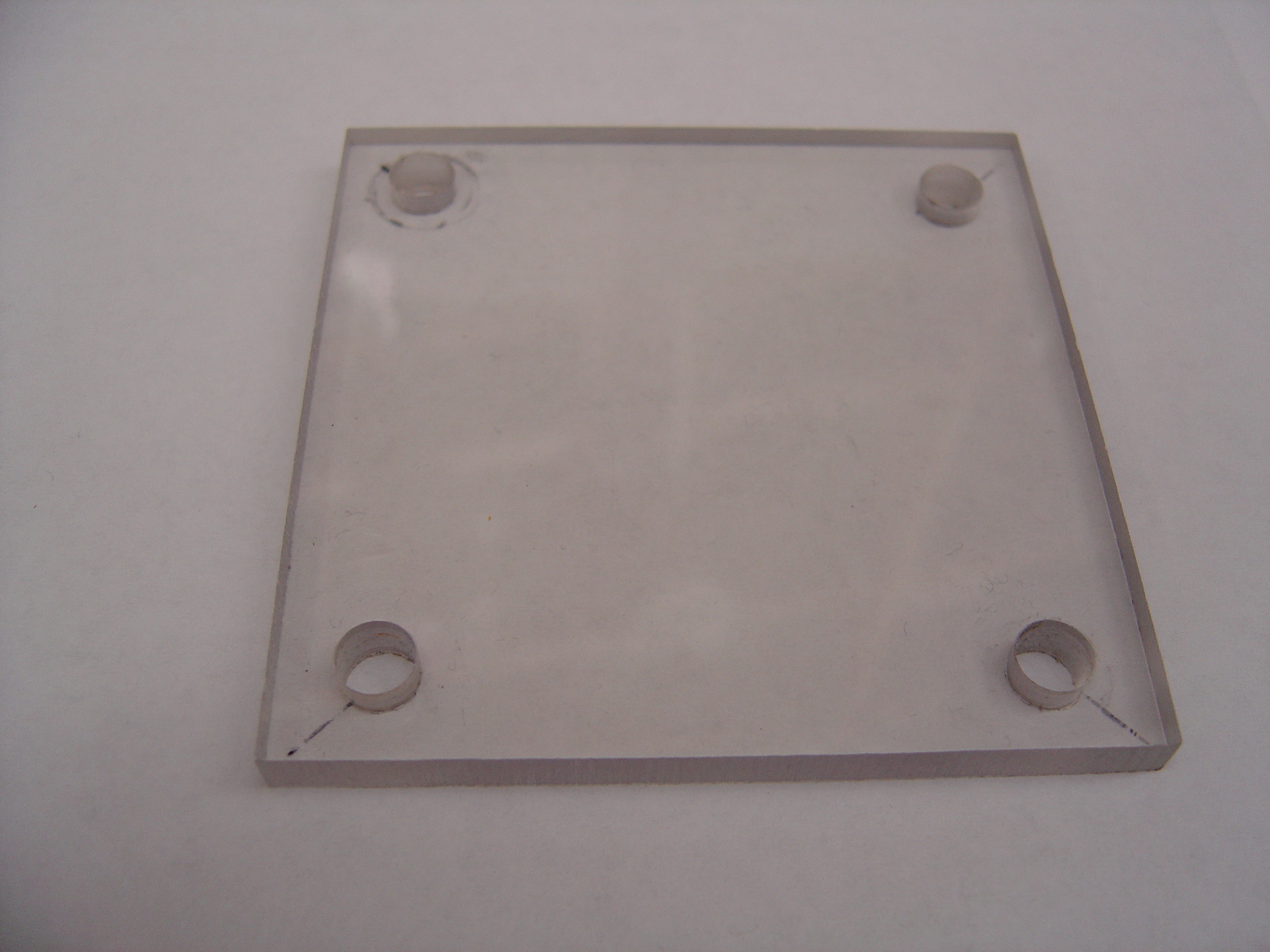
- Cut polycarbonate sheet into 4 equal 3" x 3" pieces
- Drill four 3/8" holes through each piece, 1 per corner, indented 5mm from both sides
- Cut polycarbonate tube into two equal 2" halves
- Drill four 1/4" holes through each half in configuration shown

- Tap each hole with 1/4" -28 spiral tap
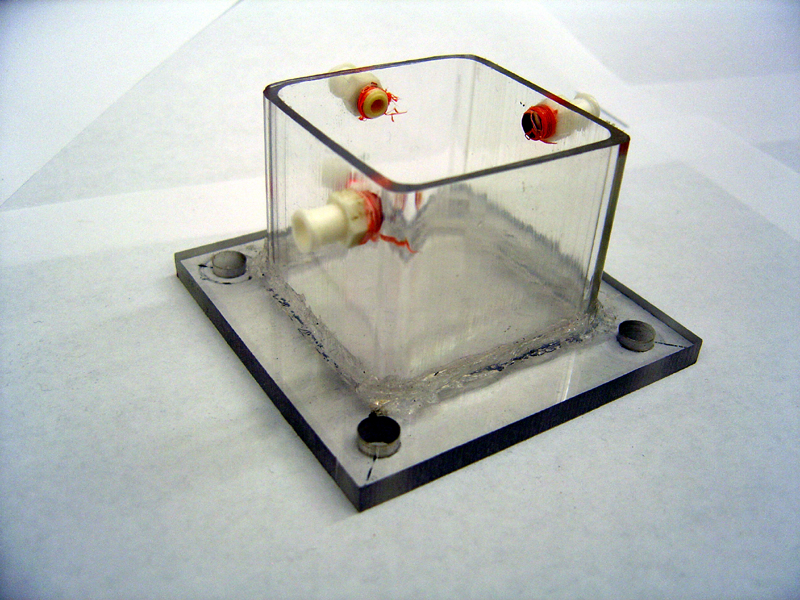
- 2) Glue Chambers (repeat for each half)
- Center tube on endplate by marking plate with 'X' from corner to corner
- Squirt 2mm thick line of silicone on edge of tube (edge furthest from holes)
- Press tube firmly against marked location on endplate
- Quickly spread excess silicone along edge
- Let stand 24h to harden
- 3) Construct Gaskets
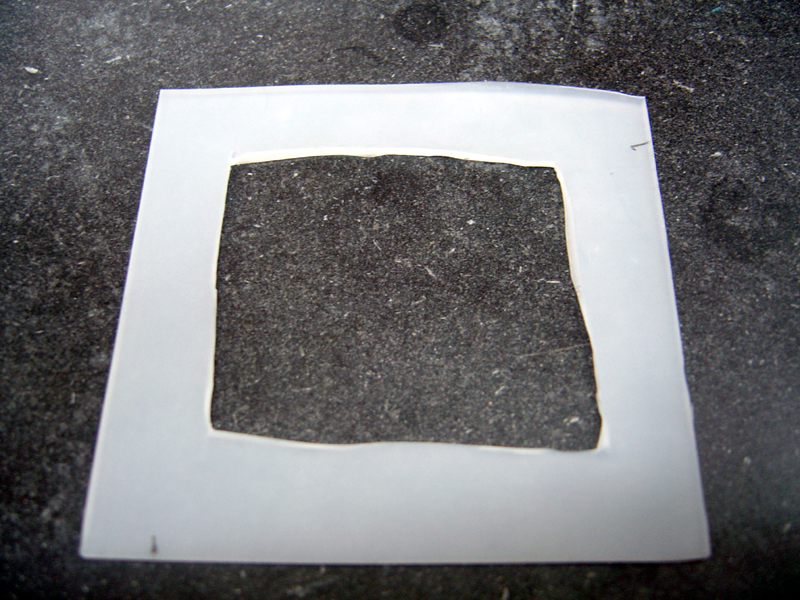
- Cut silicone sheet into two equal 2.25" x 2.25" pieces
- Cut out centered inner squares in each piece, 1.75" x 1.75"
- Using inner squares, cut two 'O' rings, inner diameter 1/4", outer diameter 1/2"
- 4) Construct Electrodes
- Cut titanium wire into one 8" piece and one 16" piece
- Using pliers, shape anode and cathode as shown
Anode Frame
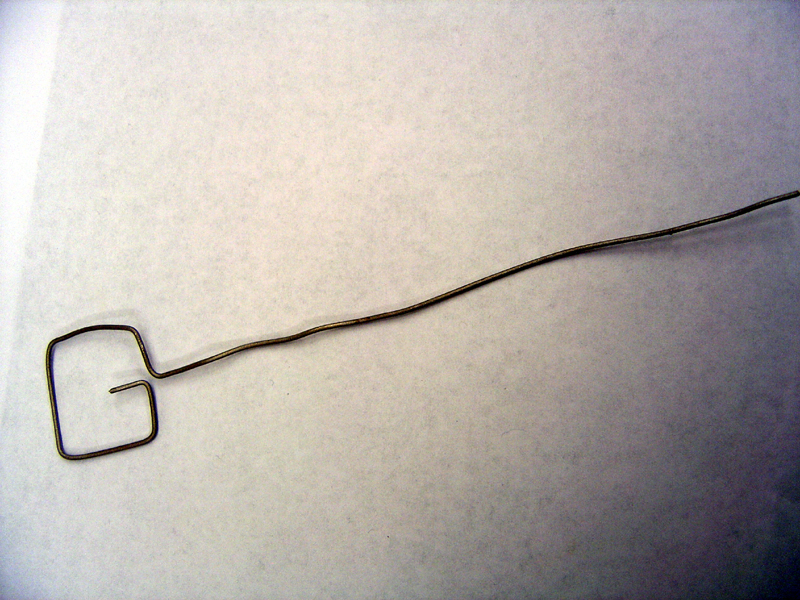
Cathode Frame
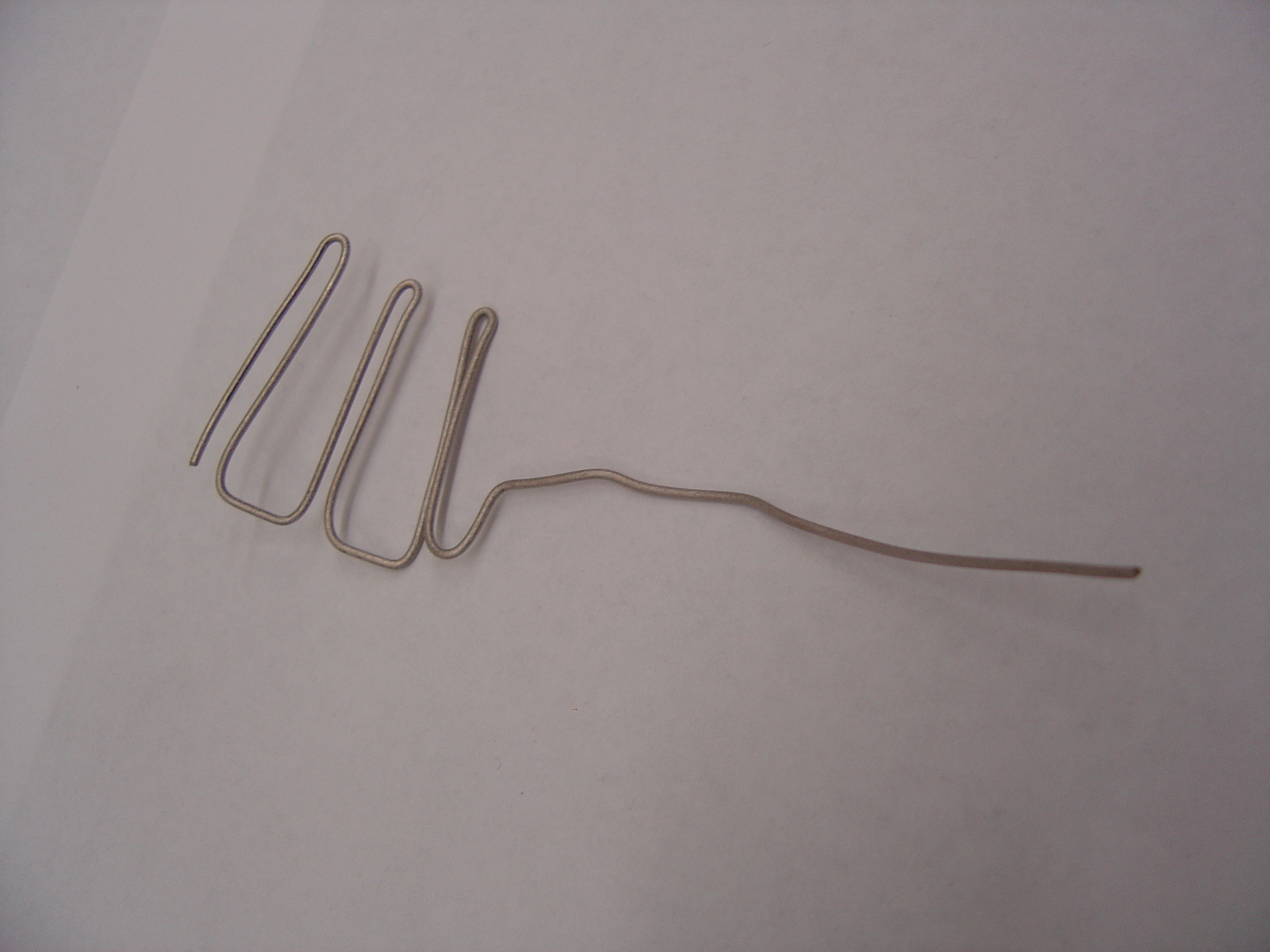
- Spear carbon felt with tip of anode titanium wire and wedge into frame
- Weave platinum carbon cloth through cathode titanium wire
Anode

Cathode
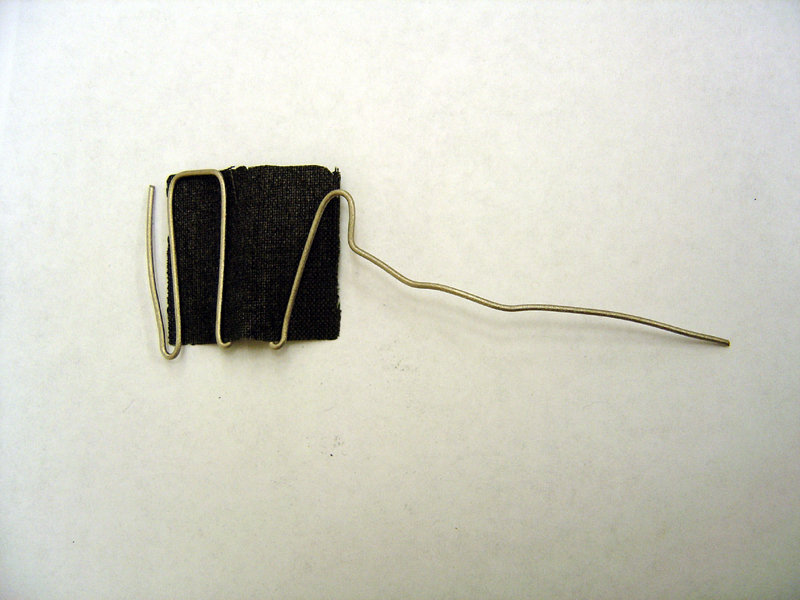
- 5) Seal Injection Ports
- Wrap threads of all eight Luer Lock screws with 1' of teflon tape in opposite direction of screwing
- Screw Luer Locks into all tapped holes in both chambers
Setup of Measurement Device
Materials
- Keithley 2700 Digital Multimeter
- Keithley 7700 Multiplexer
- Small Breadboard
- Supply of insulted thin copper wire
- 470 Ohm resistors (one/fuel cell)
Procedure
- Wire Multiplexer
- Open multiplexer, note channels
- Cut two wire 18" wire leads per fuel cell
- Strip ends, place one wire in each screw terminal, screw tight
- Tape paired wires (two are attached to each channel) near non-attached ends and label
- Clamp wire bundles near back of device with provided plastic latch clamps
- Close Multiplexer and slide into 2700 DMM
- Create Resistor Array
- Connect resistors across middle of breadboard (one per fuel cell)
- Connect leads from multiplexer across resistors (one pair across each resistor)
Controlling the DMM with LabVIEW™
- Initialize Multimeter
- Attach 2700 to COM1 port of desktop computer w/ LabVIEW™
- Download our LabVIEW™ source code MFCs.vi
- Open Program in LabVIEW™, adjust block diagram as necessary
Experiment Preparation
Begin 1 day prior to experiment
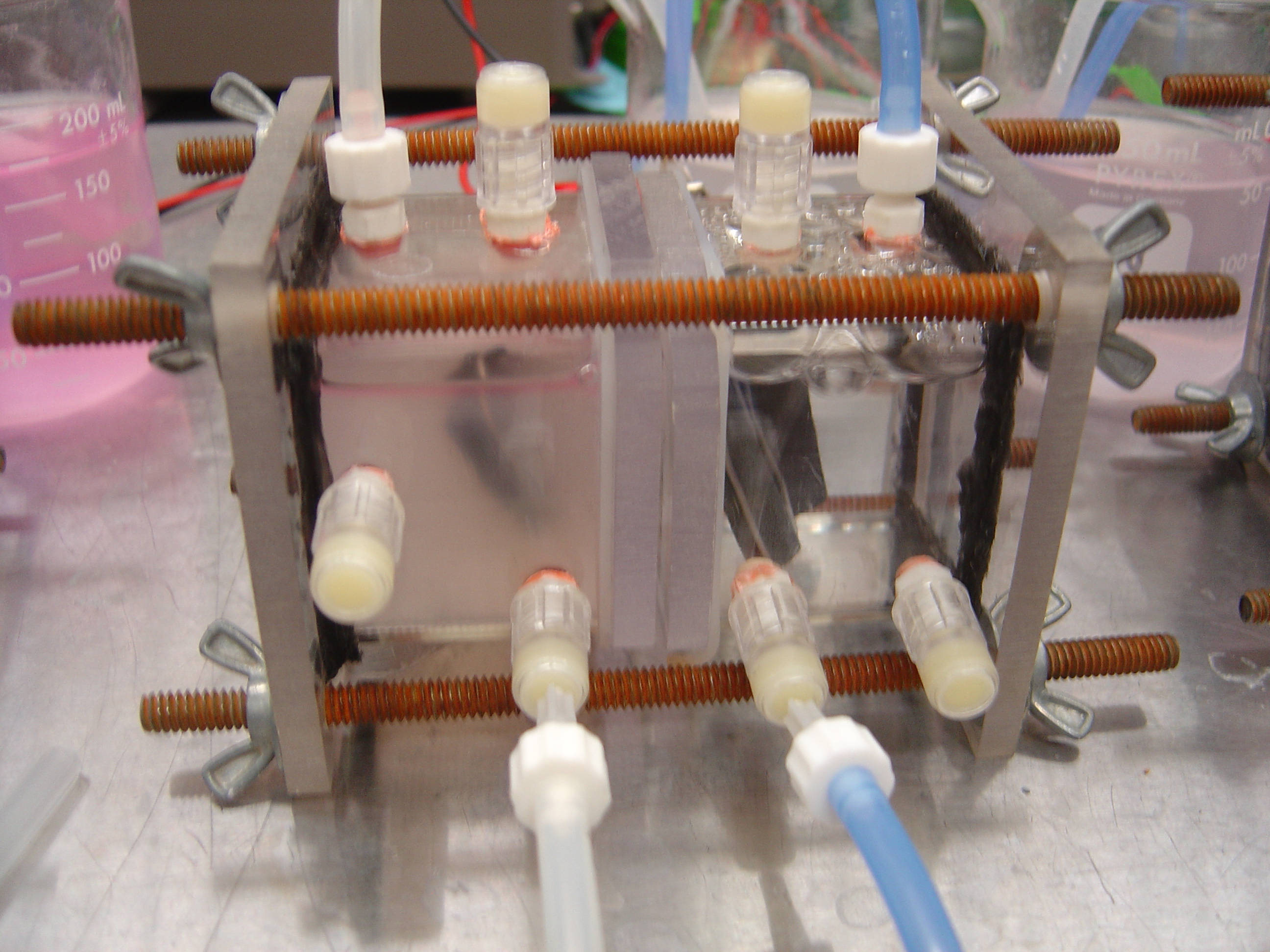
Assembling Chambers
Procedure
- Prepare Electrodes
- Attach Luer Lock injection ports to all chamber screws
- Poke tip of electrodes through designated ports from the inside

- Align Chambers
- Lay one chamber on a flat surface
- Place silicone square ring on top edge of tube
- Place polycarbonate square on silicone
- Place silicone 'O' ring around central pore
- Place Nafion membrane on top of 'O' ring
- Sandwich membrane between second 'O' ring
- Align second polycarbonate square on top of 'O' ring
- Center second silicone square ring on polycarbonate square
- Set second chamber on top of silicone, ensuring ports facing same direction as first chamber
- Clamp Chambers
- Move assembly into vice or clamp
- Insert rods through holes in end plates and screw on wing nuts
- Tighten evenly
Solutions Prep
- Chamber media (150ml / fuel cell)
- 5.844 g/L 100mM NaCl
- 15.1185 g/L 50mM PIPES (hydrogen)
- 7.0 pH
- Phosphate buffer (60ml / fuel cell)
- 2.918 g/L Monosodium phosphate, monohydrate
- 4.095 g/L Disodium phosphate, anhydrous
- 5.844 g/L 100mM NaCl
- 7.0 pH
Gas Tubing Assembly
Materials
- 25' Silicone Soft Rubber Tubing, 3/32" ID, 7/32" OD, 1/16" Wall
- Tank of Compressed Nitrogen
- Gas Regulator
- Lab Supply of Air
- 4 Plastic Luer Lock Coupling Nylon, Male X Barb, for 3/32" Tube
- 4 Plastic Luer Lock Coupling Nylon, Female X Barb, for 3/32" Tube
- Plastic Luer Lock Coupling Nylon, T junctions, for 3/32" Tube
- Syringe needles - 27 gauge
- 2 Aspirator Flasks
- 2 Rubber Stoppers
Procedure
- Make Flow Regulators
- Insert nozzle of female Luer Lock into rubber stoppers (poke hole if necessary)
- Cap aspirator flasks with rubber stoppers
- Attach tubing from gas sources to each glass nozzle of aspirator flask

- Make Manifolds
- Attach T-junction Luer Lock pieces into manifold (1 junction/ fuel cell ; 2 manifods total)
- Turn last juction such that off is facing end of manifold
- Attach tubing from stopper of flow regulators to beginning of each mainfold

Growing Strains
Materials
- 150mL LB / strain
- 1000mL airating flask / strain
- Antibiotics (if desired strain must be selected for)
- Plate or Glycerol Stock with desired strain
Procedure
- Fill flasks with LB
- Add correct concentration of selection antibiotic
- Pick single colony from plate, add to flask
- Incubate overnight in shaker at 30C
Runtime
Begin 2 hours prior to experiment
Bacteria
Procedure
- Pipet the cells out of the flask and into 250mL centrifuge containers
- Make sure the containers are close in weight (within 0.5g of each other)
- For a culture more than 250mL split it into two containers
- Set the centrifuge temperature to 22-23C, spin speed to 5000RPM, and time to 15 min
- After first spin, drain each container of the LB, making sure to leave the bacteria pellet intact
- Resuspend pellet in 50mL of potassium buffer
- After bacteria are fully resuspended (no pellets at all), spin down again, 22-23C, 5000RPM, 15min.
- Pour potassium buffer out of container slowly.
- Resuspend pellet in 50mL of potassium buffer
- Spin down again, 22-23C, 5000RPM, 15min.
- Pour potassium buffer out of container slowly.
- Resuspend pellet in 4 mL of sodium pipes
- Check OD (100microliters in 15mL or 1:150 dilution)
- If OD is in linear range, calculate dilution for desired quantity of bacteria in 1mL (typically 10^8 cells /mL of chamber media)
- Repeat dilution if not in linear range (using different ratio)
- Inject 1mL of bacteria into each chamber (see below)
Fuel Cells
Procedure
- Pipet 75mL of NaPiPES solution into each side of fuel cells
- Inject 1mL of Resazurin solution into each side of chamber
- Using Luer Lock nozzles, connect tubing from top ports to beaker w/ distilled water
- Cut tubing to span distance from manifolds to each fuel cell (1 from nitrogen, 1 from air)
- Attach syringe needles to tubing via Luer Lock nozzles
- Using Luer Lock nozzles, connect tubing from manifolds
- Start gas flow
- Poke needles through bottom ports on fuel cells
Measurements
Procedure
- Turn on computer and digital multimeter
- Open LabVIEW program
- Click Run arrow to take resistance readings
- Connect fuel cells to resistor array via alligator clips
- Click "Begin Current Readings" Icon on instrument display
Injections/Variables
Procedure
- Once current readings reach equilibrium, inject bacteria into fuels cells using syringes
- Allow bacteria to consume any carbon sources left in their media (approximately 12 hours)
- Once current levels reach stable baseline, inject 1ml lactate solution
- Inject additional variables as desired
Clean Up
Procedure
- Drain chambers and soak in 70% ethanol
- Remove carbon felt from anodes and discard (save titanium)
- Scrub all parts in ethanol and distilled water successively
- Use pipe cleaners on ports and tubes
|