Team:Heidelberg/Notebook/Killing II/6thweek
From 2008.igem.org
Revision as of 21:29, 28 October 2008 by Pascal.kraemer (Talk | contribs)


6th week
Contents |
Monday 09/08/2008
pSB1A3-Receiver-Colicin cloning
- sequencing results of pSB1A3 - Receiver cloning -> cloning not sucessful
- Primer sequences controlled
[back]
Tuesday 09/09/2008
pSB1A3-Receiver-Colicin cloning
- Amplification of Colicins
10 µl 5x Phusion HF Buffer 1.0 µl 10mM dNTPs 2.5 µl colE1/E9_prot_fw_BamHI 2.5 µl colE1_kil_prot_rv_SpeI/colE9_plasmid_rv_SpeI/colE9_lys_prot_rv_SpeI 2.0 µl Template DNA 0.5 µl Phusion DNA Polymerase 31.5 µl H2O ------- 50.0 µl
- Amplification of Receiverpart (T9002) without GFP
10 µl 5x Phusion HF Buffer 1.0 µl 10mM dNTPs 2.5 µl T9002_lux_pR_fw_XbaI 2.5 µl T9002_lux_pR_rv_SpeI_BamHI_RBS 2.0 µl Template DNA (T9002 plasmid) 0.5 µl Phusion DNA Polymerase 31.5 µl H2O ------- 50.0 µl
program:
98 °C 30 sec 98 °C 10 sec 59 °C 30 sec 72 °C 45 sec 72 °C 5 min 4 °C constant
HisTag cloning of Colicins for purification
- Amplification of Colicins
10 µl 5x Phusion HF Buffer 1.0 µl 10mM dNTPs 2.5 µl colE1/E9_prot_fw_BamHI 2.5 µl colE1_prot_rv_HindIII/colE9_prot_rv_XmaI 2.0 µl Template DNA 0.5 µl Phusion DNA Polymerase 31.5 µl H2O ------- 50.0 µl
program:
98 °C 30 sec 98 °C 10 sec 59 °C 30 sec 72 °C 45 sec 72 °C 5 min 4 °C constant
[back]
Wednesday 09/10/2008
pSB1A3-Receiver-Colicin cloning
- Gelextraction Receiver PCR: 0.7% Agarose, 100 V, 60 min
- Gelextraction Receiver PCR: QIAquick Gel Extraction (Qiagen), eluted in 30 µl H2O
- Digestion of Receiver & pSB1A3 with SpeI and XbaI, 1 h 30 min 37 °C -> 10 min 65°C
20 µl DNA 6 µl H2O 4 µl NEBuffer 2 3 µl SpeI (NEB) 3 µl XbaI (NEB) 4 µl BSA 10x ----- 40 µl
- Gelextraction of pSB1A3 digestion: 0.7 % Agarose, 50 min, 135 V
- Gelextraction of pSB1A3 digestion: QIAquick Gel Extraction (Qiagen), eluted in 30 µl H2O, cutted pSB1A3 and T9002 -> not used
- Ligation of pSB1A3 and Receiver: 1 h RT, 10 min 65 °C
10 µl Receiver DNA 2 µl Vector DNA pSB1A3 (sapped, from last week) 2 µl Buffer T4 DNA Ligase (Fermentas) 2 µl T4 DNA Ligase (Fermentas) 4 µl H2O Nuclease Free (Fermentas) ----- 20 µl
- Transformation of prior ligation: 5 µl per 50 µl E. coli MG1655
- start thawing 50 µl competent E. coli MG1655 cells on crushed ice - add 5 µl ligation - incubate on ice for 20 minutes - heat shock at 42 °C for 45 sec - incubate 2 min on ice - add 200 µl preheated LB media - incubate at 37 °C for 1 h, 300 rpm - Plate 200 µl on preheated LB-Amp plate - incubate overnight
- Controllgels of Colicin Amplification (Tuesday 080909): 1% Agarose, 37 min, 135V
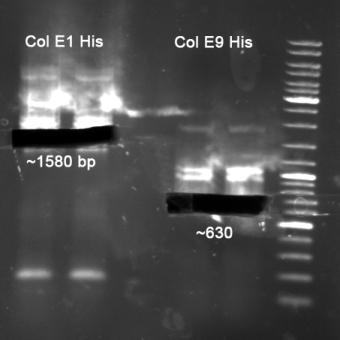
- Colicin E1 & E1 for HisTag
- Colicin E9 lys & E9 for HisTag
- Colicin E9 whole plasmid
- Colicin E1 & E1 for HisTag
HisTag cloning of Colicins for purification
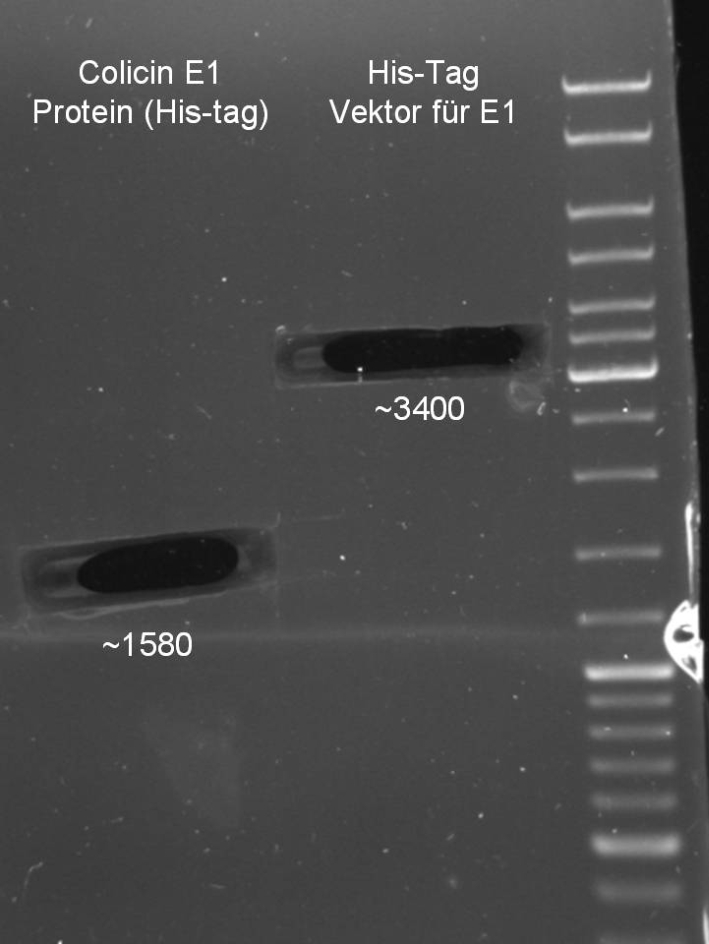
- Gelextraction of Colicin E1 and Colicin E9 PCR: 0,7%, 60 min, 100 V
- Gelextraction of Colicin E1 and Colicin E9 PCR: QIAquick Gel Extraction (Qiagen), eluted in 30 µl H2O
Colicin activity test
- inocculation of liquid cultures from glycerolstocks, incubation over night at 37 °C
-10 ml M9 media + 10 µl Kanamycin + E. coli Top10 with I20260 -10 ml M9 media + 10 µl Ampicilin + E. coli MG1655 with pKC67 (Colicin E9 plasmid) -10 ml M9 media + 10 µl Ampicilin + E. coli MG1655 with pKC67 (Colicin E9 plasmid) -10 ml M9 media + 200 µl 10% Glucose + E. coli JC411 (Colicin E1 plasmid) -10 ml M9 media + 200 µl 10% Glucose + E. coli JC411 (Colicin E1 plasmid) -10 ml LB media + 200 µl 10% Glucose + E. coli JC411 (Colicin E1 plasmid) -10 ml LB media + 200 µl 10% Glucose + E. coli JC411 (Colicin E1 plasmid)
[back]
Thursday 09/11/2008
pSB1A3-Receiver-Colicin cloning
- Digestion of pSB1A3 with XbaI and SpeI from cloning cultures of last week. We used these cultures because the bande where easier to distinguish. !!!
- 2008-09-18 UPDATE: the different bands were probably from the religated pSB1A3 plasmid, which cannot be cutted with XbaI and SpeI. -> No cutted pSB1A3 backbone
- Gelextraction of pSB1A3: 0.7 % Agarose, 1 h, 135 V
- Gelextraction of pSB1A3: QIAquick Gel Extraction (Qiagen), eluted in 30 µl H2O
- Overnight ligation of pSB1A3(gelextraction, Thursday 2008-09-11) and T9002_without_GFP (gelextraction, Wednesday 2008-09-10)
10 µl Receiver DNA 2 µl Vector DNA pSB1A3 2 µl Buffer T4 DNA Ligase (Fermentas) 2 µl T4 DNA Ligase (Fermentas) 4 µl H2O Nuclease Free (Fermentas) ----- 20 µl
- Gelextraction colicin E1, colicin E9, colicin E9 lysis genes: 0.7 % Agarose, 1 h, 135 V
- Gelextraction colicin E1, colicin E9, colicin E9 lysis genes: QIAquick Gel Extraction (Qiagen), eluted in 40 µl H2O -> stored ad -20 °C
HisTag cloning of Colicins for purification
- Miniprep of pQE-30 plasmids from ON-culture(QIAprep Spin Miniprep Kit (250), Qiagen)
- Digestion of pQE-30 (E1 -> HindIII, E9 -> XmaI)
20 µl DNA 6 µl H2O 4 µl NEBuffer 2/4 3 µl BamHI (NEB) 3 µl HindIII/XmaI (NEB) 4 µl BSA 10x ----- 40 µl
- Digestion of Colicin E1/E9 (E1 -> HindIII, E9 -> XmaI)
20 µl DNA 6 µl H2O 4 µl NEBuffer 2/4 3 µl BamHI (NEB) 3 µl HindIII/XmaI (NEB) 4 µl BSA 10x ----- 40 µl
- Gelextraction of pQE-30 and Colicin E1 digestion: 0.7 % Agarose, 1 h, 135 V
- Gelextraction of pQE-30 and Colicin E9 digestion: 0.7 % Agarose, 1 h, 135 V
- Gelextraction of pQE-30 and Colicins digestion: QIAquick Gel Extraction (Qiagen), eluted in 30 µl H2O
- Overnight ligation:
10 µl Colicin DNA 2 µl Vector DNA pQE-30 2 µl Buffer T4 DNA Ligase (Fermentas) 2 µl T4 DNA Ligase (Fermentas) 4 µl H2O Nuclease Free (Fermentas) ----- 20 µl
Colicin activity test
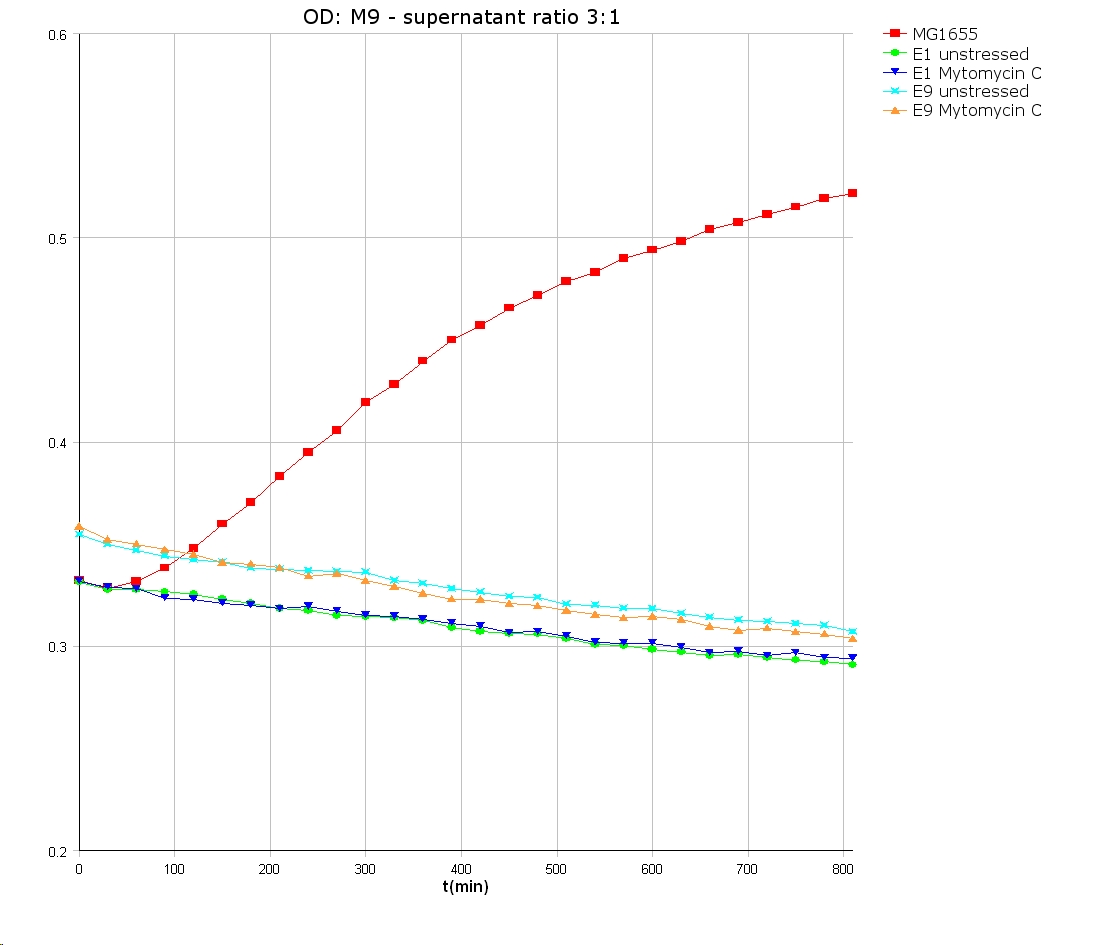
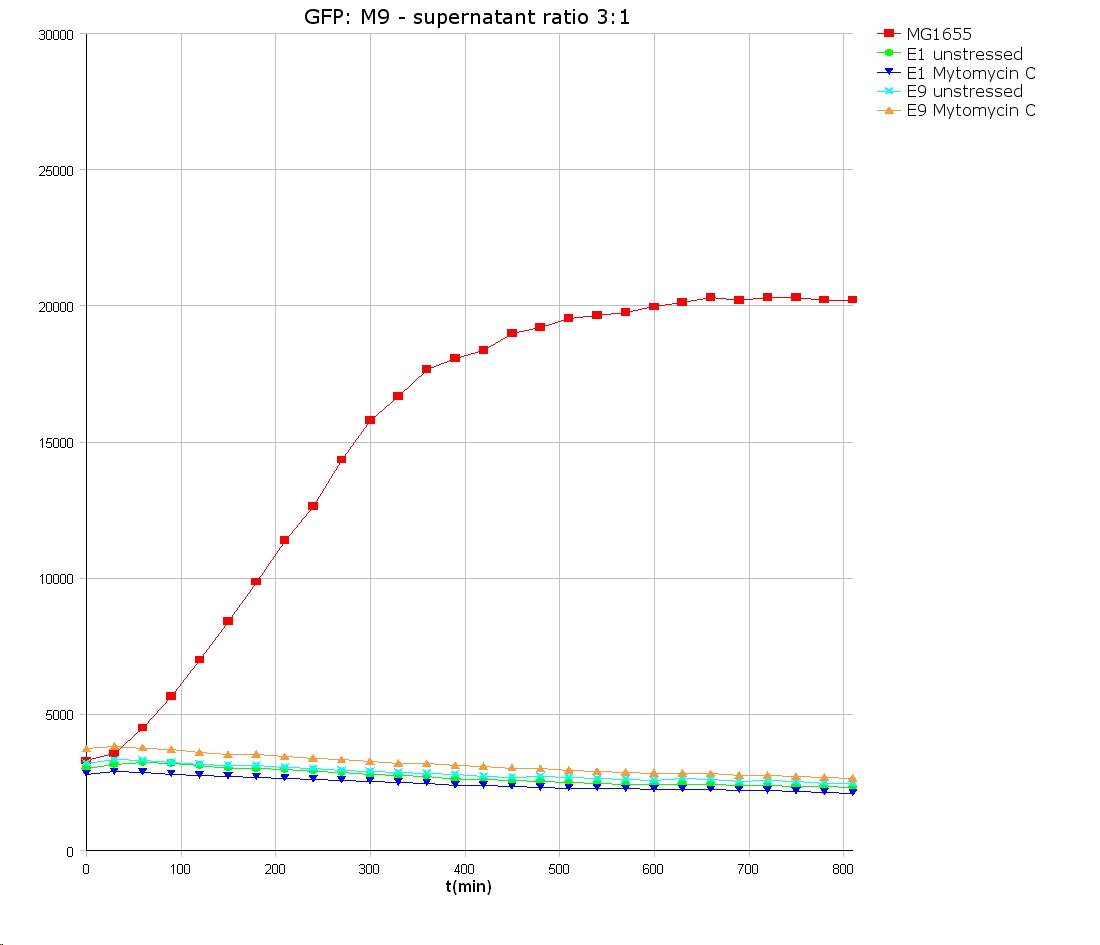
- 9 am: One E9 - M9 culture, one E1 - M9 culture and one E1 - LB culture were stressed with 10 µl of 0.1 mg/ml Mytomycin C
- 4 pm: The colicin E1 LB cultures were thrown away, because the grew well in M9. The MG1655 and colicin cultures were sonicated in a ultrasonic bath three time for 15 seconds. After sonication cell extract was observed under microscope -> lot of dead cells -> succesfull lysis.
- Overnight-Activity-Assay:
- lysed cells were centrifuged for 15 min at 400 rpm. The supernatants were transferd to new falcons and were used for a colicin activity test.
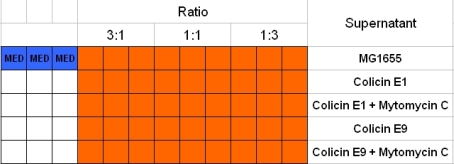
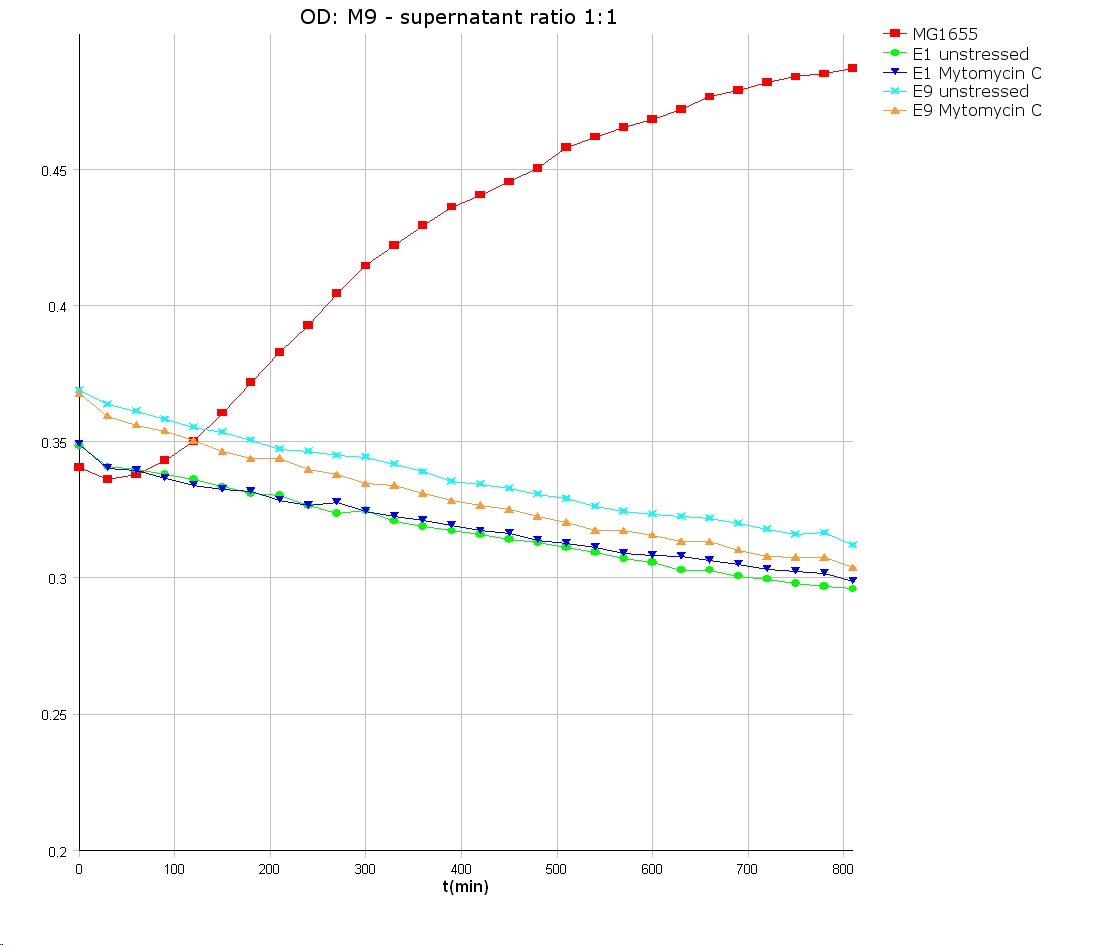
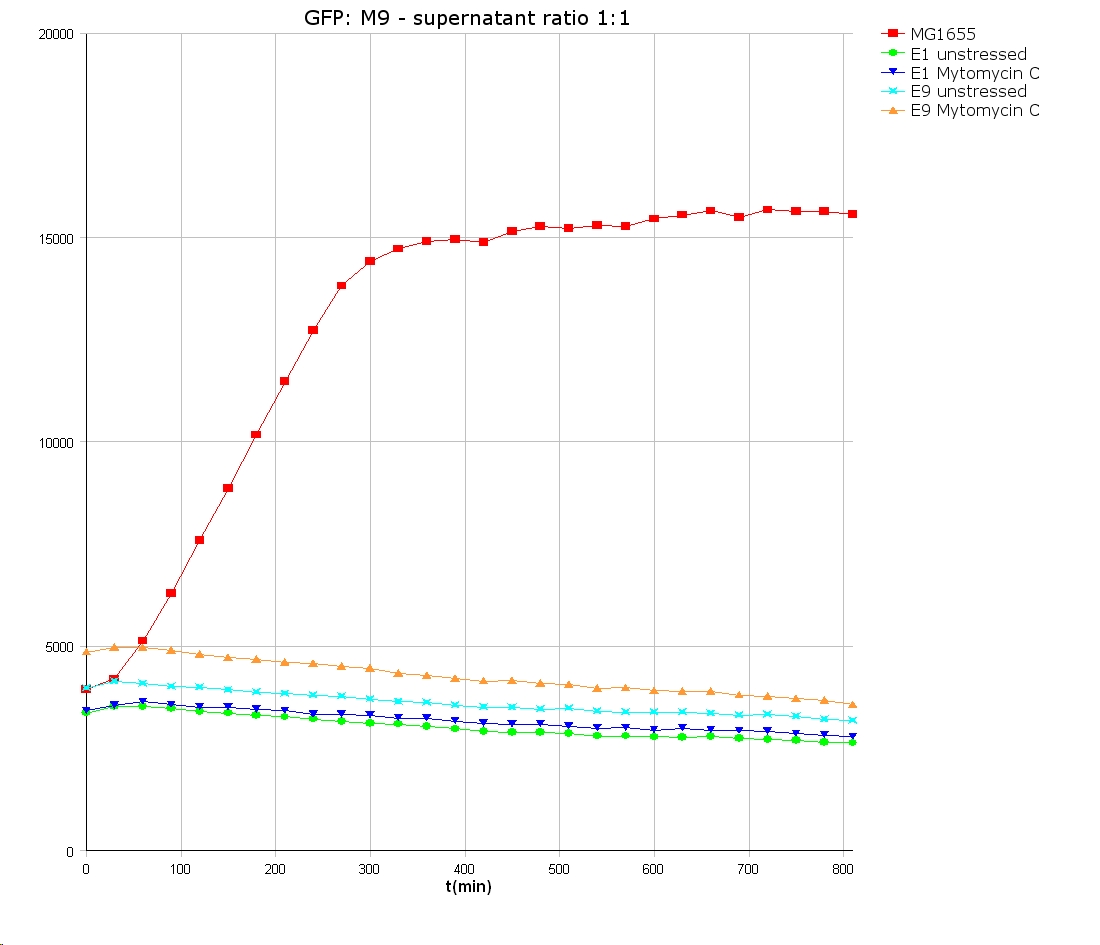
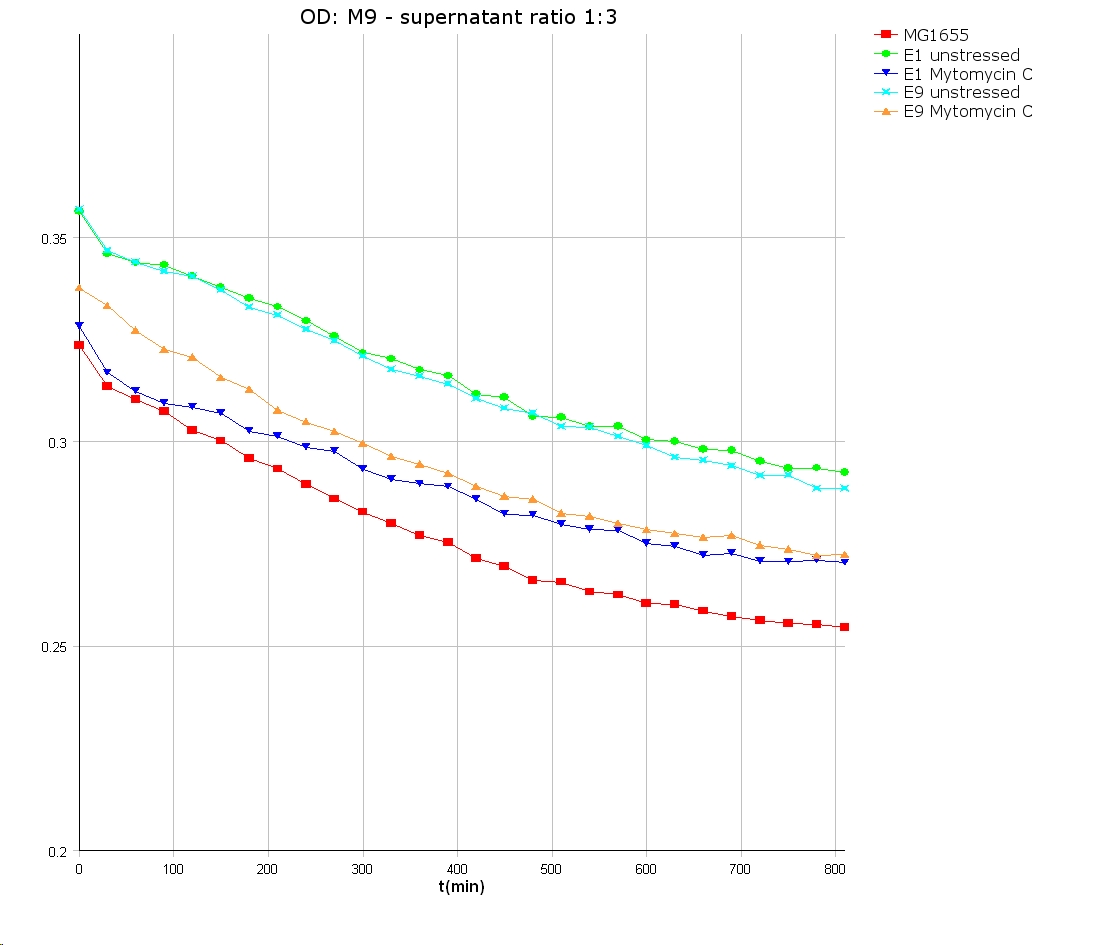
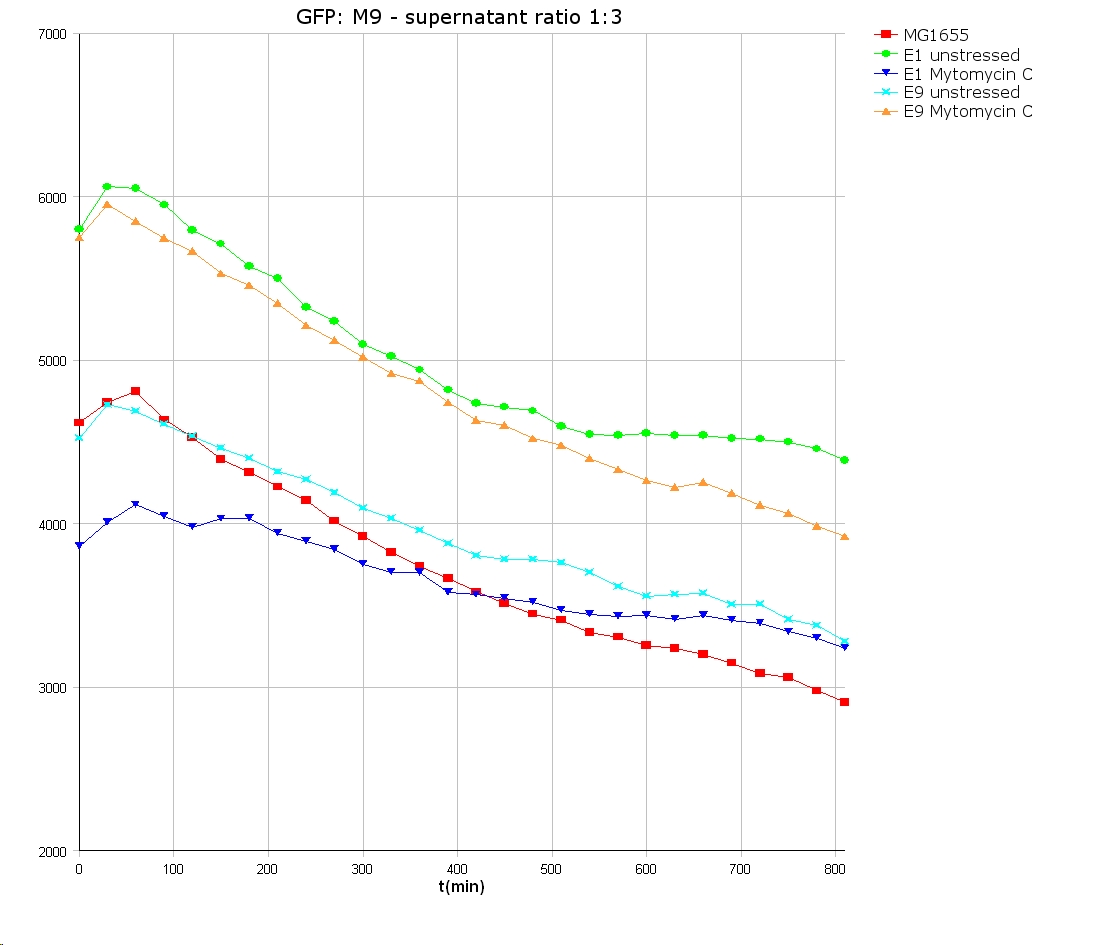
- well plate scheme:
3:1 -> 3 ml M9 media + 1 ml supernatant + 200 µl reference promotor cells (I20260) 1:1 -> 2 ml M9 media + 2 ml supernatant + 200 µl reference promotor cells (I20260) 1:3 -> 1 ml M9 media + 3 ml supernatant + 200 µl reference promotor cells (I20260)
- ON-measurement for 14 h at 37 °C in Tecan well plate reader
Friday 09/12/2008
pSB1A3-Receiver-Colicin cloning
- New ligation because controll gel didn't show any bands for pSB1A3 -> probably a too low concentration: : 1 h 40 min RT, 15 min 65 °C
10 µl Receiver DNA 6 µl Vector DNA pSB1A3 (sapped, from last week) 2 µl Buffer T4 DNA Ligase (Fermentas) 2 µl T4 DNA Ligase (Fermentas) ----- 20 µl
- Transformation of ligation (Tuesday 2008-09-11) into E. coli TOP 10 cells: 5 µl per 50 µl E. coli BL21 cells
- start thawing 50 µl competent E. coli TOP 10 cells on crushed ice - add 5 µl ligation - incubate on ice for 20 minutes - heat shock at 42 °C for 45 sec - incubate 2 min on ice - add 200 µl preheated LB media - incubate at 37 °C for 1 h, 300 rpm - Plate 200 µl on preheated LB-Amp plate - incubate overnight at 37 °C
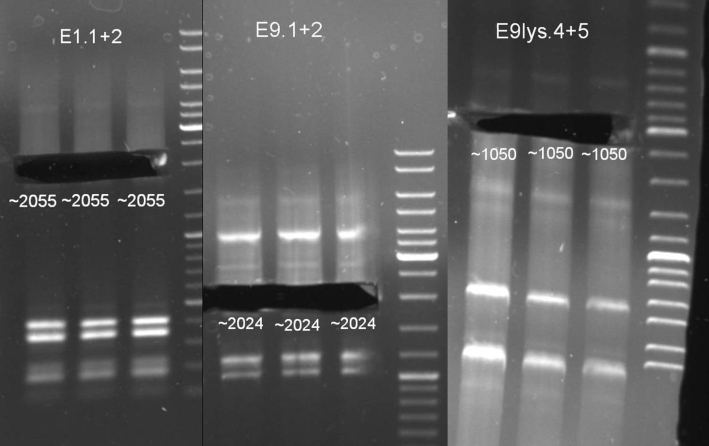
- Controllgel of PCR: 1% Agarose, 30 min, 135 V
HisTag cloning of Colicins for purification
- Transformation of ColE1 and ColE9 into BL21 cells: 5 µl per 50 µl E. coli BL21 cells
- start thawing 50 µl competent E. coli BL21 cells on crushed ice - add 5 µl ligation - incubate on ice for 20 minutes - heat shock at 42 °C for 45 sec - incubate 2 min on ice - add 200 µl preheated LB media - incubate at 37 °C for 1 h, 300 rpm - Plate 200 µl on preheated LB-Amp plate - incubate overnight at 37 °C
Colicin activity test
- Colicin E1 and E9 kills other E. coli cells efficient
Other
- Maxiprep of I15030 and T9002 for plasmid backbone. (Compact Prep Plasmid Maxi Kit (25), Qiagen)
Saturday 09/13/2008
pSB1A3-Receiver-Colicin cloning
- Transformation results: we picked and plated 8 colonies of each transformation on LB-Ampicilin plates and in addition we inocculated liquid cultures.
HisTag cloning of Colicins for purification
- Transformation results: We plated our transformation on LB-Kanamycin because BL21 cells contain a helper plasmid which has an Kanamycin resistance. But pQE-30 plasmids have an Ampicilin resistance. Because of that we transderd colonies from an bacteria lawn to Ampicilin/Kanamycin plates.
Sunday 09/14/2008
pSB1A3-Receiver-Colicin cloning
- Miniprep of pSB1A3-Receiver-Cloning from ON-cultures(QIAprep Spin Miniprep Kit (250), Qiagen)
- Digestion of pSB1A3-Rec XbaI and SpeI, 1h 30 min -> 37 °C, 15 min -> 65 °C
10 µl DNA 3 µl H2O 2 µl NEBuffer 2 1.5 µl XbaI (NEB) 1.5 µl SpeI (NEB) 2 µl BSA 10x ------ 20 µl
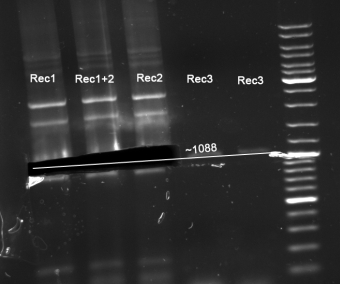
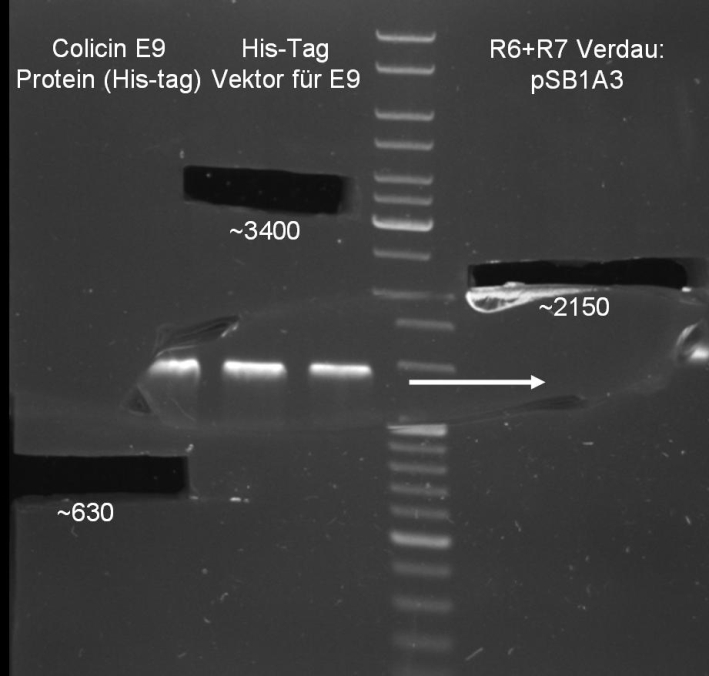
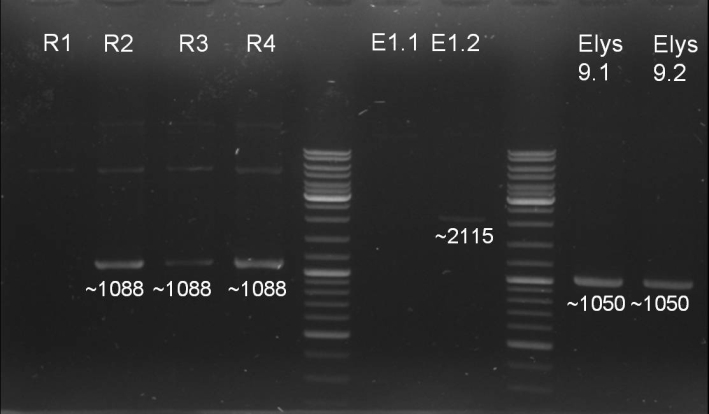
- Gel for selection of positive clones: Estimated fragments ~2150 bp pSB1A3 backbone and ~1088 bp T9002 without GFP. No bands at 1088 bps. Colony-PCR-Screen on Mondayday.
- Since selection of positive clones failed, we started a new ligation
- Sapping: 30 min 37°C, 10 min 65°C
34 µl pSB1A3 (digested) 4 µl SAP Buffer 1 µl SAP Enzyme ----- 39 µl
- Ligation of pSB1A3 and T9002 without GFP: 18 h 16 °C, 20 min 65 °C, 4 °C const
6 µl Receiver DNA 4 µl Vector DNA pSB1A3 2 µl Buffer T4 DNA Ligase (NEB) 2 µl T4 DNA Ligase (NEB) 6 µl H2O ----- 20 µl
HisTag cloning of Colicins for purification
- Plated colonies from Saturdayurday (2008-09-13) were not grown.
- Retransformation of ligation:
- ColE1His: 2.5 µl ligation on 50 µl BL-21 cells, 10 µl ligation on 100 µl TOP 10 cells
- ColE9His: 2.5 µl ligation on 50 µl BL-21 cells, 10 µl ligation on 100 µl TOP 10 cells
- New Ligation with other insert/plasmid ration
- Ligation of pQE-30 and Colicins: 18 h 16 °C, 20 min 65 °C, 4 °C const
9 µl ColE1/E9 His 3 µl Vector DNA pQE-30 2 µl Buffer T4 DNA Ligase (NEB) 2 µl T4 DNA Ligase (NEB) 4 µl H2O ----- 20 µl
 "
"