Team:Heidelberg/Notebook/Sensing Group/Notebook/11thweek
From 2008.igem.org
Revision as of 19:56, 26 October 2008 by Koljaschleich (Talk | contribs)


Contents |
Monday, 10/13/2008
- Miniprep of LuxS_mut_EcoRI and F1-YFP, F2-YFP
- Digestion of Fusion-YFP with BamHI (NEBuffer3 + BSA)
- Insert: 5937 bp and 1998 bp
- No Insert: 7202 bp
- Digestion of LuxS with EcoRI/NdeI (NEBuffer EcoRI)
- Insert: 2773 bp and 1862 bp
- 4635 bp
- Fusion-PCR for LuxS standardization with Phusion
- 30s @ 98°C || 10s @ 98°C | 30s @ 55°C | 1min @ 72°C || 5min @ 72°C | 4°C (30 cycles)
Tuesday, 10/14/2008
- PCR Purification, Digestion of LuxS and pSB2K3 with EcoRI/PstI (EcoRI buffer + BSA). 1h @ 37°C
- Fusion PCR for LuxS with Phusion and Annealing Temperature Gradient (50-60°C)
- Transformation of Fusion-YFP into MG1655
Wednesday, 10/15/2008
- PCR Purification of LuxS_standard and F1-GeneArt, F2-GeneArt
- Digestion of LuxS and Fusion Constructs with EcoRI/PstI (NEBuffer EcoRI + BSA)
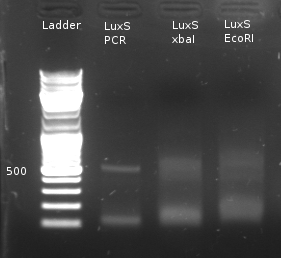
- Digestion of LuxS with EcoRI/xbaI (NEBuffer2 + BSA) to check for restriction sites
- Cloning into pSB2K3
- Test Digestion of LuxS with EcoRI and xbaI to test if mutation was successful
- Digestion of Fusion1-YFP and Fusion2-YFP constructs with BamHI
- Inoculation of 50µl Fusion-YFP O/N culture in 5mL LB. Incubation for 2h @ 37°C. Induction of Fusion-YFP cells with 0.01% Arabinose for 3h. Visualization under microscope
- Expression of Fusion-Receptor positive for both constructs. Even Localization to the membrane can be seen in some cells.
 "
"