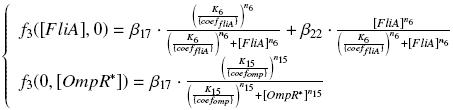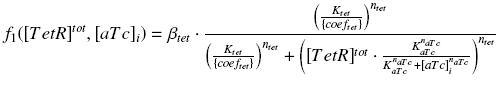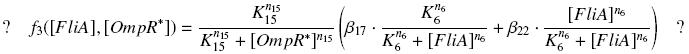|
Method & Algorithm : ƒ1
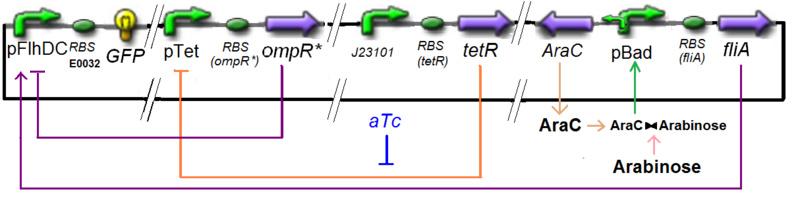 Specific Plasmid Characterisation for ƒ3 We have [OmpR*]real = {coefomp}expr(pTet) = {coefomp} ƒ1([aTc]i)
and [FliA]real = {coefFliA}expr(pBad) = {coefFliA} ƒ2([arab]i)
but we use [aTc]i = Inv_ƒ1( [OmpR*] )
and [ara]i = Inv_ƒ2( [FliA] )
So, at steady-states,
↓ Table ↑
| param
| signification
| unit
| value
| comments
|
| (fluorescence)
| value of the observed fluorescence
| au
|
| need for 20 values with well choosen [aTc]i
|
| conversion
| conversion ration between
fluorescence and concentration
↓ gives ↓
| nM.au-1
| (1/79.429)
|
|
| [GFP]
| GFP concentration at steady-state
| nM
|
|
|
| γGFP
| dilution-degradation rate
of GFP(mut3b)
↓ gives ↓
| min-1
| 0.0198
| Only dilution :
Time Cell Division : 35 min.
|
| ƒ1
| activity of
pTet with RBS E0032
| nM.min-1
|
|
|
| param
| signification
corresponding parameters in the equations
| unit
| value
| comments
|
| βtet
| basal activity of
pTet with RBS E0032
β16
| nM.min-1
|
|
|
| (Ktet/{coeftetR})
| activation constant of TetR><pTet
K13
| nM
|
| The optimisation program will give us (γ Ktet / {coeftet} ƒ0)
The literature [?] gives Ktet =
|
| ntet
| complexation order of TetR><pTet
n13
| no dimension
|
| The literature [?] gives ntet =
|
| KaTc
| complexation constant aTc><TetR
K12
| nM
|
| The literature [?] gives KaTc =
|
| naTc
| complexation order aTc><TetR
n12
| no dimension
|
| The literature [?] gives naTc =
|
|
↓ Algorithm ↑
|
find_ƒ1
function optimal_parameters = find_f1(X_data, Y_data, initial_parameters)
function output = expr_pTet(parameters, X_data)
for k = 1:length(X_data)
output(k) = parameters(1) * (1 - ...
hill((1 - hill(X_data(k),parameters(4),parameters(5))),parameters(2),parameters(3)));
end
end
options=optimset('LevenbergMarquardt','on','TolX',1e-10,'MaxFunEvals',1e10,'TolFun',1e-10,'MaxIter',1e4);
optimal_parameters = lsqcurvefit( @(parameters, X_data) expr_pTet(parameters, X_data), ...
initial_parameters, X_data, Y_data, 1/10*initial_parameters, 10*initial_parameters, options );
end
Inv_ƒ1
function quant_aTc = Inv_f1(inducer_quantity)
global gamma, f0;
function equa = F(x)
equa = f1( (f0/gamma) , x ) - inducer_quantity;
end
options=optimset('LevenbergMarquardt','on','TolX',1e-10,'MaxFunEvals',1e10,'TolFun',1e-10,'MaxIter',1e4);
quant_aTc = fsolve(F,1,options);
end
|
Also, this experiment will enable us to know the expression of ƒ1 :
<Back - to "Implementation" |
<Back - to "Protocol Of Characterization" |
| param
| signification
| unit
| value
| comments
|
| [expr(pFlhDC)]
| expression rate of
pFlhDC with RBS E0032
| nM.min-1
|
| need for 20 mesures with well choosen values of [aTc]i
and for 20 mesures with well choosen values of [arab]i
and 5x5 measures for the relation below?
|
| γGFP
| dilution-degradation rate
of GFP(mut3b)
| min-1
| 0.0198
|
|
| [GFP]
| GFP concentration at steady-state
| nM
|
| need for 20 + 20 measures
and 5x5 measures for the relation below?
|
| (fluorescence)
| value of the observed fluorescence
| au
|
| need for 20 + 20 measures
and 5x5 measures for the relation below?
|
| conversion
| conversion ratio between
fluorescence and concentration
| nM.au-1
| (1/79.429)
|
|
| param
| signification
corresponding parameters in the equations
| unit
| value
| comments
|
| β13
| production rate of FliA-pFlhDC with RBS E0032
β13
| nM.min-1
|
|
|
| (K12/{coeffliA})
| activation constant of FliA-pFlhDC
K12
| nM
|
|
|
| n12
| complexation order of FliA-pFlhDC
n12
| no dimension
|
|
|
| β2
| production rate of OmpR-pFlhDC with RBS E0032
β2
| nM.min-1
|
|
|
| (K22/{coefomp})
| activation constant of OmpR-pFlhDC
K22
| nM
|
|
|
| n22
| complexation order of OmpR-pFlhDC
n22
| no dimension
|
|
|
Then, if we have time, we want to verify the expected relation
|
 "
"