Team:BrownTwo/Limiter/utility
From 2008.igem.org
The utility of threshold limitationMuch as excessive signal levels can destroy a radio transmitter, excessive genetic expression can cause damage in living systems. While cells are usually well-equipped to modulate their own transcriptional behavior, an investigation into pathological gene expression indicates that many situations arise wherein the expected regulatory response fails. Our device is designed to augment endogenous gene regulatory pathways. While one can surely identify numerous cases of abnormal gene expression in cellular systems, we turn our attention to one particular network related to a crucial decision in cell fate, apoptosis.
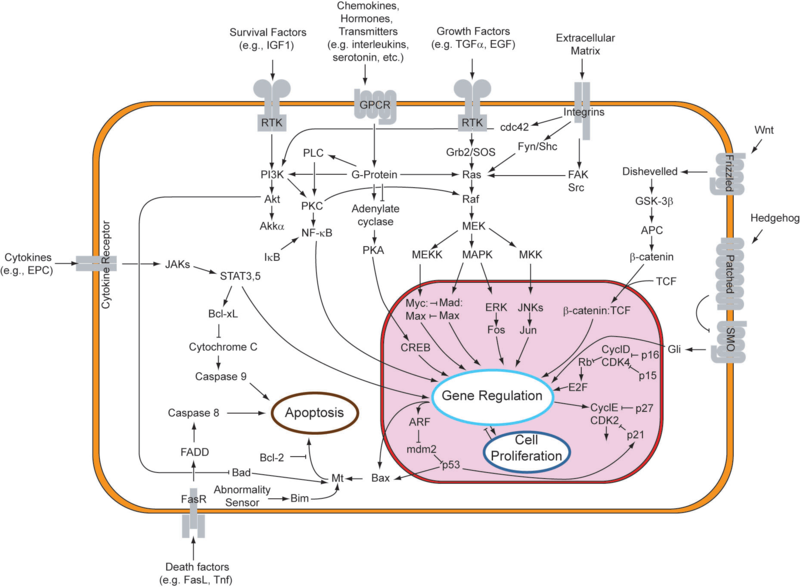
Cellular suicide and the cancer phenotypeIn response to either stressful circumstances or communication from neighbors, cells will often undergo a highly regulated process that results in cell death, known as apoptosis. Pro-apoptotic and anti-apoptotic factors expressed in a viable cell strike an ongoing balance that codes for cell survival. Such a balance tilts in favor of an apoptotic cell fate in the presence of appropriate stimuli, like oxidative stress or UV treatment. In multicellular organisms, programmed cell death plays a healthy role in development and disease regulation. One might say that the system of apoptosis evolved to provide individual cells a method of succumbing to the greater good of the whole organism. As can be observed in the figure below, the genetic underpinnings that encode for apoptosis are truly immense. An apoptotic response generally entails changes to multiple nodes in this robust network of genes. One important point to note is that apoptosis can be triggered extrinsically, from factors in the cell's environment, or intrinsically, when the cell recognizes that it should not continue to proliferate. Due to its complexity, the system is still being explored, especially for its potential involvement in cancer. Given the function of apoptosis in defining cell fate, it should come as no surprise that multiple disease states are characterized by an inactive or reduced apoptotic response. When apoptosis cannot proceed, often due to mutations in the genes responsible for initiating the pathway, cell proliferation can occur unchecked. This allows for propagation of mutated DNA as well as an abnormal accumulation of cells. This phenotype is highly characteristic of tumor formation in multicellular organisms. In designing a system , one might desire
Through an intricate balance between oncogenes, which are described for their ability to result in a cancer phenotype when overexpressed
With respect to its role in the cancer phenotype, two theories dominate the discussion about the relationship between
check out references 32-35 and 38-40 for more info on how IAPs relate to malignancies check our references 41-43 for more info about how XIAP confounds proapoptotic signals -could conceivably use the up-limiter to increase XIAP expression -alternatively, could up-regulate the factors that cause Smac/DIABLO and Omi/HtrA2 release from the mito Another possible target for down-regulation is Bcl-xL, which prevents the release of cytochrome c into the cytoplasm -activated by STAT3,5 Interestingly, XIAP is turned off by the expression of Bax and Bak (which inhibit Smac) and it inhibits capsase 3 while Bcl-xL is inhibited by caspase 3 and in turn inhibits Bax and Bak (Science signaling) -thus, inhibition of XIAP will result in inhibition of Bcl-xL via caspase 3, both of which will result in promoting apoptosis. Could also target Bax or Bak, since high levels of both will also result in decrease in levels of XIAP and Bcl-xL -might even want to target both, seeing as they operate at different levels of the apoptosis pathway (Bcl-xL before cytochrome c release and XIAP afterwards) Apoptosis in yeastWhile still an ongoing debate, there are increasingly more signs that yeast undergo. Despite the fact that there seems, that yeast undergo a similar programmed cell death
ResearchReferences
|
 "
"