From 2008.igem.org
Recipes
- Tris-Cl, 1M
- Dissolve 121g Tris base in 800ml H2O
- Adjust to desired pH with concentrated HCl
- Mix and add H2O to 1 liter
- (Approximately, 20ml HCl for pH 7.4 and 42ml for pH 8.0)
- EDTA, 0.5M (pH 8.0)
- Dissolve 186.1g Na2 EDTA-2H2O in 700ml H20
- Adjust pH to 8.0 with 10M NaOH(~50ml)
- Add H2O to 1 liter
- Breaking buffer - 100ml
- 2ml Triton X-100
- 1ml Sodium dodecyl sulfate (SDS)
- 0.5844g NaCl (100mM)
- 1ml 1M Tris-Cl pH 8.0 (10mM)
- 200uL 0.5M EDTA (1mM)
Primers
PGK promoter
Fwd:5'TTTT GAATTC AAAGATGCCGATTTGGGCGC
Rev:5'TTTT GAGCTC GTTTTATATTTGTTGTAAAA
PGK terminator
Fwd:5'TTAT GGGCCC GAAATAAATTGAATTGAATT
Rev:5'TTTTG AAGCTT CAGCTTTAACGAACGCAGA
Ste2
Fwd:5'GCCC TCTAGA ATGTCTGATGCGGCTCCTTC
Rev:5'TTAT GGGCCC TCATAAATTATTATTATCTT
Fus1 upstream
Fwd:5'GTGG GAATTC TAATAATCAGAACTCCAACA
Rev:5'GGCG TCTAGA TTTGATTTTCAGAAACTTGA
Fus1 downstream:
Fwd:5'GCGA GGTACC TGAAAATAATATTGACGTTC
Rev:5'TTAT GCGGCCGC TATTCACCAGACCCGCTCCT
22nd July
- Yeast obtained from Dr. Zhao
24th July
- Prepared liquid culture for DNA extraction
- Made 1M Tris. Cl pH 8.0
- Made 4M ammonium acetate
22nd August
- Attempted DNA extraction of W303a genomic DNA
- Protocol from Wiley's Current Protocols in Molecular Biology
- Result: Failed to finish protocol
- Obtained more yeast from Dr. Zhao
25th August
- Prepared overnight culture for DNA extraction (3:27pm)
26th August
- Attempted DNA extraction
- Prepped overnight culture
27th August
- Performed PCR: PGK Terminator
| Buffer G
| 12.5uL
| x4
| 50uL
|
| Forward Primer
| 0.5uL
| x4
| 2uL
|
| Reverse Primer
| 0.5uL
| x4
| 2uL
|
| H2O
| 10.8uL
| x4
| 43.2uL
|
| Taq
| 0.2uL
| x4
| 0.8uL
|
| template
| 0.5ul
|
| Negative control
| 3 H2O
|
- PCR program:
- 4 min 94 degrees
- 25-30x 30s 94 degrees
- 30s Tm primers
- 1 min/KB 72 degrees
- 7 min 72 degrees
- For the gel: 5uL loading dye gel is in cold room
- Prepped 3 overnight cultures
28th August
- Extracted DNA from 4 cultures
- Ran gel of PCR products (1.5% agarose, 200V)
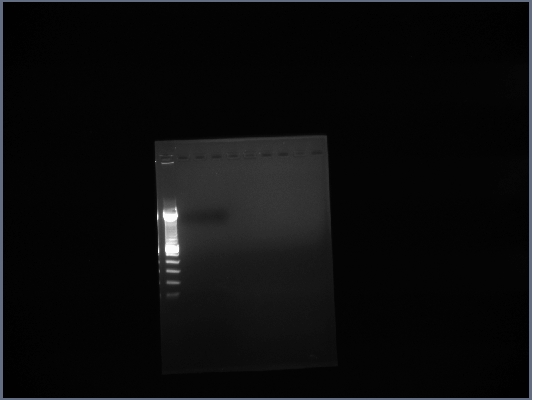
2nd September
| 5 PRIME Mastermix
| 10uL
| x3
| 30uL
|
| Forward Primer
| 0.5uL
| x3
| 1.5uL
|
| Reverse Primer
| 0.5uL
| x3
| 1.5uL
|
| Template
| 10uL
| x3
| 30uL
|
| H2O
| 10.8uL
| x4
| 43.2uL
|
| Negative control
| 25uL H2O
|
3rd September
- Ran gel
- Ladder lane 7
- Sample 7 spilled
- 1% agarose
- 120V
- 50 minutes
- Poor results--no bands present
8th September
| 5PRIME Mastermix
| 10uL
| x3
| 30uL
|
| Forward Primer
| 0.5uL
| x3
| 1.5uL
|
| Reverse Primer
| 0.5uL
| x3
| 1.5uL
|
| Template
| 14uL
| x3
| 42uL
|
- Prepped 4 overnight cultures
9th September
- Signs of life in 3 of the cultures
- Ran gel on PCR from 8th September--no bands present
- 150V, 50 minutes
- No sign of DNA
- Ladder from Courtney
10th September
- Split culture
- Ran gel from 8th September again--no bands present
- 150V, 50 minutes
- 0.75% gel
- Ladder from Courtney
11th September
| 5 PRIME Mastermix
| 10uL
| x3
| 30uL
|
| Forward Primer
| 0.5uL
| x3
| 1.5uL
|
| Reverse Primer
| 0.5uL
| x3
| 1.5uL
|
| Template
| 14uL
| x3
| 42uL
|
12th September
- Isolated genomic DNA from 8 cultures of W303a yeast cells
- Protocol from Wiley's Current Protocols in Molecular Biology
- Labeled templates 1,2,3,4 and 1a,2a,3a,4a
- Ran gel of PCR from 11th September
15th September
- PCR PGK Promotor
- Finnzymes Phusion High Fidelity DNA Polymerase
| 5x Phusion HF Buffer
| 10uL
| x3
| 30uL
|
| 10mM dNTPs
| 1uL
| x3
| 3uL
|
| Primer A(Forward)
| 1uL
| x3
| 3uL
|
| Primer B(Reverse)
| 1uL
| x3
| 3uL
|
| Template 1
| 10uL
| x3
| 30uL
|
| Phusions DNA polymerase
| 0.5uL
| x3
| 1.5uL
|
| H2O
| 26.5uL
| x3
| 79.5uL
|
18th September
- Ran reaction mentioned on 15th September
- Extracted DNA from gel from 8th September (PGK Terminator)
| Tube
| Gel(g)
|
| 1
| 0.332
|
| 2
| 0.278
|
| 3
| 0.307
|
| 4
| 0.349
|
| 5
| 0.385
|
19th September
- Gel of PGK Promotor from 15th September has no DNA present
23rd September
- PCR: Fus1 Downstream
- EPICENTRE Bioetechnologies - MasterAmp(TM) Taq DNA Polymerase
| MasterAmp Taq 10x PCR Buffer
| 5uL
|
| 1mM dNTPs
| 1uL
|
| Primer 1
| 0.5uL
|
| Primer 2
| 0.5uL
|
| 25mM MgCl2
| 2uL
|
| Taq DNA Polymerase
| 0.25uL
|
| Template 2
| 20uL
|
| Water
| 20.75uL
|
- PCR Settings:
- 4mins, 94 degree celcius
- 30s, 94 degree celcius
- 30s, 5 degrees below primer melting temperature
- 1 min, 72 degree celcius -- to step 2 -- 30x
- 7 min, 72 degree celcius
24th September
- Ran gel of Fus1 Downstream from 23rd September
- Result: No DNA present on gel
25th September
| Mastermix
| 8.25uL
| x3
| 24.75uL
|
| Forward Primer
| 0.5uL
| x3
| 1.5uL
|
| Reverse Primer
| 0.5uL
| x3
| 1.5uL
|
| Template 3
| 20uL
| x3
| 60uL
|
| Water
| 20.75uL
| x3
| 62.25uL
|
- same protocol as 23rd September
- The master mix contains the buffer, dNTPs, MgCl, and Taq
- Template 3 (3 reactions run)
30th September
| Mastermix
| 8.25uL
| x3
| 24.75uL
|
| Forward Primer
| 0.5uL
| x3
| 1.5uL
|
| Reverse Primer
| 0.5uL
| x3
| 1.5uL
|
| Template 3
| 20uL
| x3
| 60uL
|
| Water
| 20.75uL
| x3
| 62.25uL
|
- same protocol as 23rd September
- Template 4 and 1a (3 reactions each)
- Gel: 1% agarose, 150V, 35 minutes -> poor results
- lanes 2,3 -> faint smear

1st October
| Mastermix
| 8.25uL
| x3
| 24.75uL
|
| Forward Primer
| 0.5uL
| x3
| 1.5uL
|
| Reverse Primer
| 0.5uL
| x3
| 1.5uL
|
| Template 3
| 20uL
| x3
| 60uL
|
| Water
| 20.75uL
| x3
| 62.25uL
|
- same protocol as 23rd September
- Templates 2a, 3a, 4a used (3 reactions each)
3rd October
- Ran Ste2 gel from 1st October
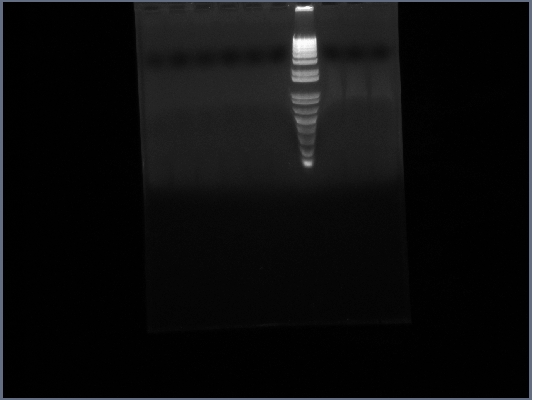
8th October
| PCR Buffer
| 5uL
|
| 10mM dNTPs
| 1uL
|
| Forward Primer
| 1uL
|
| Reverse Primer
| 1uL
|
| MgCl2
| 5uL
|
| Taq DNA Polymerase
| 0.25uL
|
| Template
| 25uL
|
| Water
| 11.75uL
|
- same protocol as 23rd September
- Use DNA extracted from gel on 18th September (5 reactions)
- Also extracted DNA from gel from 30th September (Fus1 Upstream)
9th October
- PCR: Ste2
- Protocol is the same as 23rd September
- Template used is product from 1st October (9 reactions total)
- PCR: Fus1 Upstream
- Protocal matches 23rd September
- Template used was DNA extracted from the gel from the 8th October, which came from the PCR run on 30th September (5 reactions total)
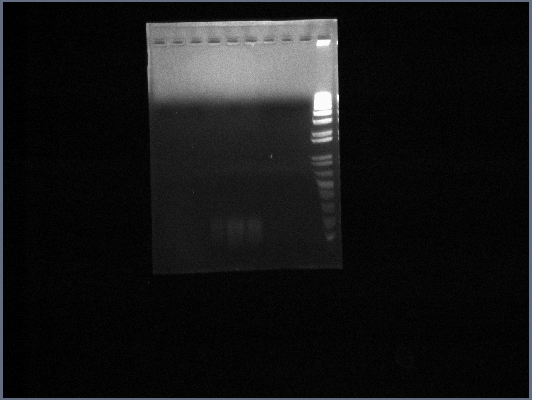
- Ran PGK terminator gel from yesterday
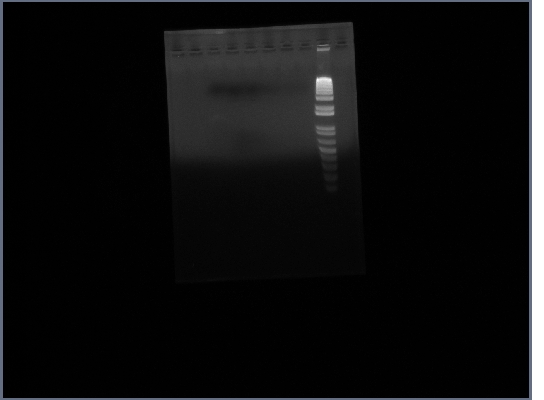
12th October
- PCR: Fus1 (3 reactions each)
- Template is products from 9th October
13th October
- Ran gel of PCR with Fus1
- Used 25uL or product, 5uL of loading dye

14th October
| MasterAmp Taq 10x PCR Buffer
| 5uL
|
| 1mM dNTPs
| 1uL
|
| Primer 1
| 0.5uL
|
| Primer 2
| 0.5uL
|
| 25mM MgCl2
| 5uL
|
| Taq DNA Polymerase
| 0.25uL
|
| Template
| 25uL
|
| Water
| 11.75uL
|
- Template is reaction from 12th October
15th October
| 5 PRIME MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 25uL
|
| Water
| 4uL
|
- Ran gel of Ste2 from 10/14

- Ran gel of Fus1 upstream from 10/14 (no ladder, oops)
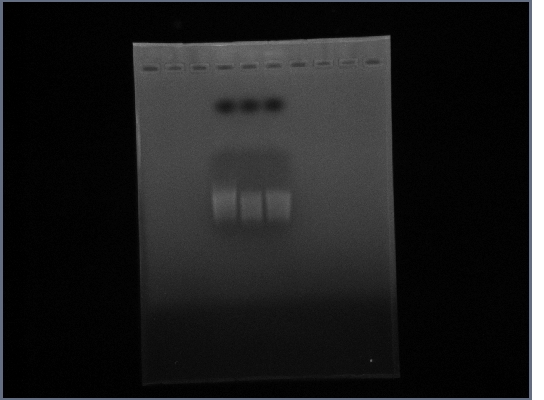
16th October
- PCR: Fus1 Downstream, PGK Promoter
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 20uL
|
| Water
| 9uL
|
- Tube 1: Template 4 - Fus1
- Tube 2: Template 4a - Fus1
- Tube 3: Template 4 - PGK Promoter
- Tube 4: Template 4a - PGK Promoter
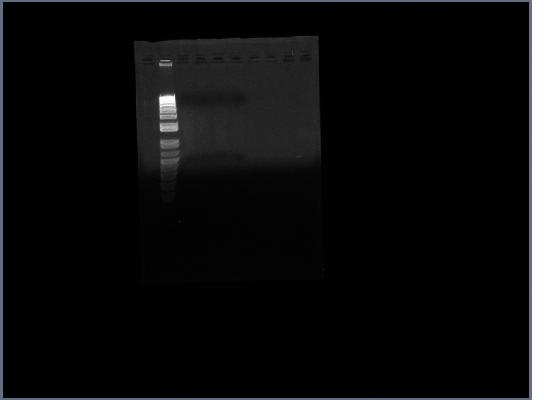
- ran Gel of PGK terminator from 10/15
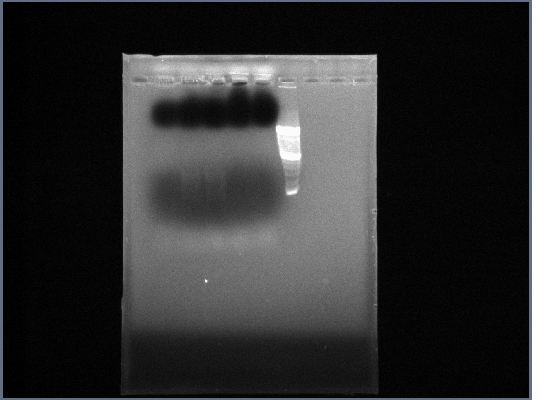
- Extracted Ste2 from 10/15 and re-amplified:
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 20uL
|
| Water
| 9uL
|
17th October
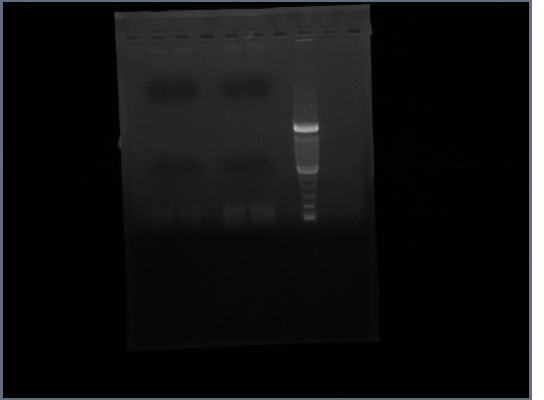
- Ran gel of Ste2 from 10/16
- Tube 1 - lane 2,3
- Tube 2 - lane 5,6
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 20uL
|
| Water
| 9uL
|
- template is products from 10/14
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 20uL
|
| Water
| 9uL
|
- Template is products from 10/15
- Extracted Ste2(Gel from today)
18th October
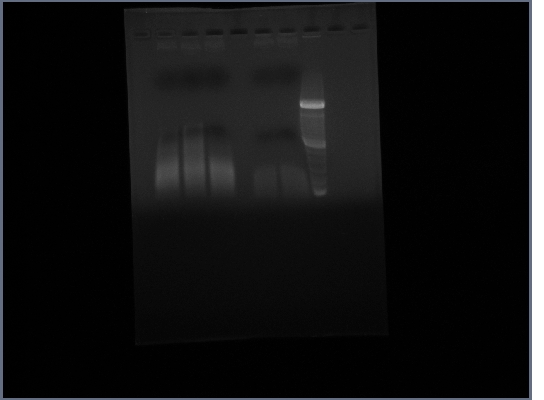
Ran gel of Fus1 upstream and PGK terminator from yesterday
20th October
- Extracting chromosomal DNA from yeast cells
- W303A - Yellow
- YPD DL
- YHP1 YPD HD - One is orange (YHP1)(Cap was removed in incubator), Other is Yellow
- Orange - YHP1 YPD HD
- Green - YHP2 YPD HD
- Pink - W303A YPA DL
- Yellow - WD303A YPD DL
21st October
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 20uL
|
| Water
| 9uL
|
- 2 tubes
- Block B of black machine
- Annealing temperature: 31 degrees celcius
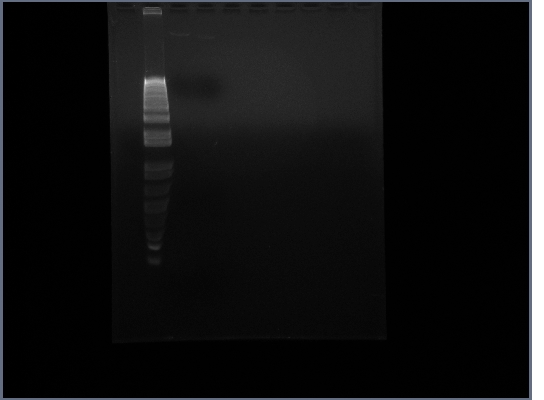
- Ran gel on Ste2 from today
22nd October
- New primers for biobricks are here
- Brought to standard concentration, 30uM
- Added 33.3uL of water per nmol primer
- PCR: Fus1 and PGK Terminator
| MasterMix
| 20uL
|
| Primer Fwd
| 0.75uL
|
| Primer Rev
| 0.75uL
|
| Template
| 25uL
|
| Water
| 3.5uL
|
- Forward and Reverse primers are new biobrick primers that arrived today
- Template is PCR product from 17th October
- A,B,C -> Fus1 Upstream -> 1,2,3
- 1,2 -> PGK Terminator -> 4,5
- Annealing temperature: 38 degrees celcius
- In freezer in yellow case
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 25uL
|
| Water
| 4uL
|
- Forward primer, Reverse Primer are new primers that arrived today
- Template is PCR product from 21st October
- Two tubes in block B
- The 3 gels in the cold room do not have EtBr
23rd October
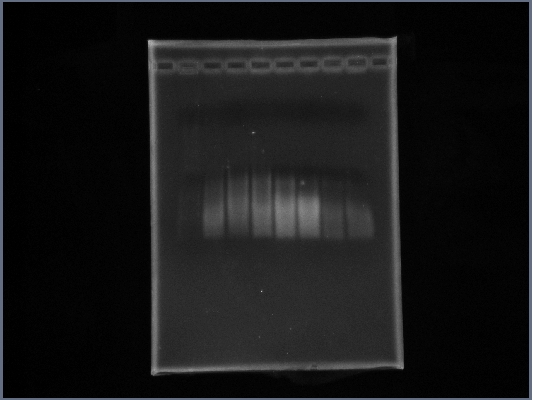
- Ran gel of Fus1 Upstream, PGK Terminator, Ste2 from yesterday
- PCR: Fus1 Downstream, PGK Promoter
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 20uL
|
| Water
| 9uL
|
- 4 reactions each of Fus1 Upstream(1,2,3,4) and PGK Promoter(A,B,C,D)
- Gel shows no bands.
- PCR: Fus1 Downstream, Fus1 Upstream, PGK Promoter, PGK Terminator, Ste2
- Template genomic DNA from 20th October (x4 different reactions)
- Used all Template
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 20uL
|
| Water
| 9uL
|
24th October
- Ran gel of Fus1 upstream(lanes 3,4,5), PGK terminator(lanes6,7), and Ste2(lanes 8,9) from 10/22
- ladder is lane 2; 1 and 10 are nothing
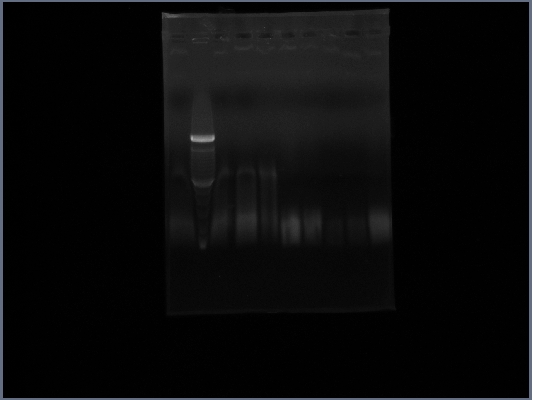
- PCR of Fus1 downstream, Fus1 upstream, PGK promoter, PGK terminator, Ste2
| MasterMix
| 20uL
|
| Primer Fwd
| 0.5uL
|
| Primer Rev
| 0.5uL
|
| Template
| 29uL
|
- The templates were the rest of the genomic DNA extracts from 9/12
- Three reactions of each gene were run
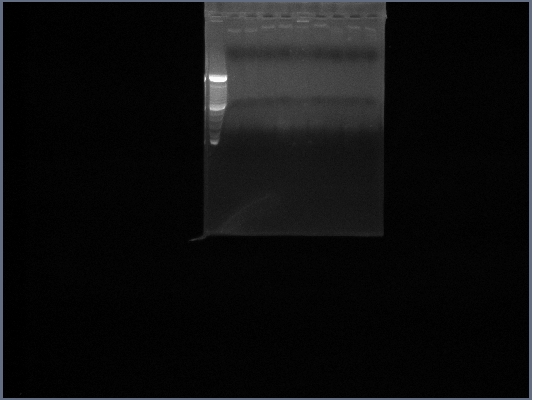
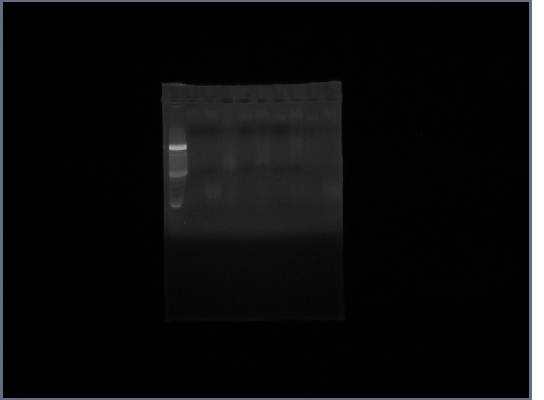
- (on left)Fus1 downstream lanes 2,3,4; Fus1 upstream lanes 5,6,7; PGK promoter lanes 8,9,10;
- (on right)PGK terminator lanes2,3,10; Ste2 lanes 4,5,6; Fus1 downstream lanes 7,8,9;
25th October
- Extracted DNA from the gel from 10/24
- FUS1 upstream from the higher bands of lanes 3 and 4
- PGK terminator from the lower bands of lanes 6 and 7
- DNA ligation
| DNA
| 5uL
|
| buffer
| 5uL
|
| Re1 (Pst1)
| 1uL
|
| Re2 (EcoR1)
| 1uL
|
| Water
| 37.5uL
|
26th October
- Incubate for 20mins at 80 degrees celcius
| Ligation Buffer
| 4uL
|
| DNA Ligase
| 1uL
|
| DNA
| 3uL
|
| Plasmid
| 9uL
|
| Water
| 3uL
|
- Let sit for 5 min.
- 5uL of above mixture to competent cells
- Heat shock 30s (42 degrees)
- Add SOC Media (200uL)
- Incubate 60 min.(37 degrees)
- Plate 200uL
- Incubate 37 degrees celcius overnight
27th October
The transformation failed; try again with the same protocol:
- Using extracts 3 and 7 (+Ligation buffers, Ligase, Plasmid, and Water)

 "
"
















