From 2008.igem.org
Things we did today
Wetlab work
Checked plasmid prep from weekend
- Grace
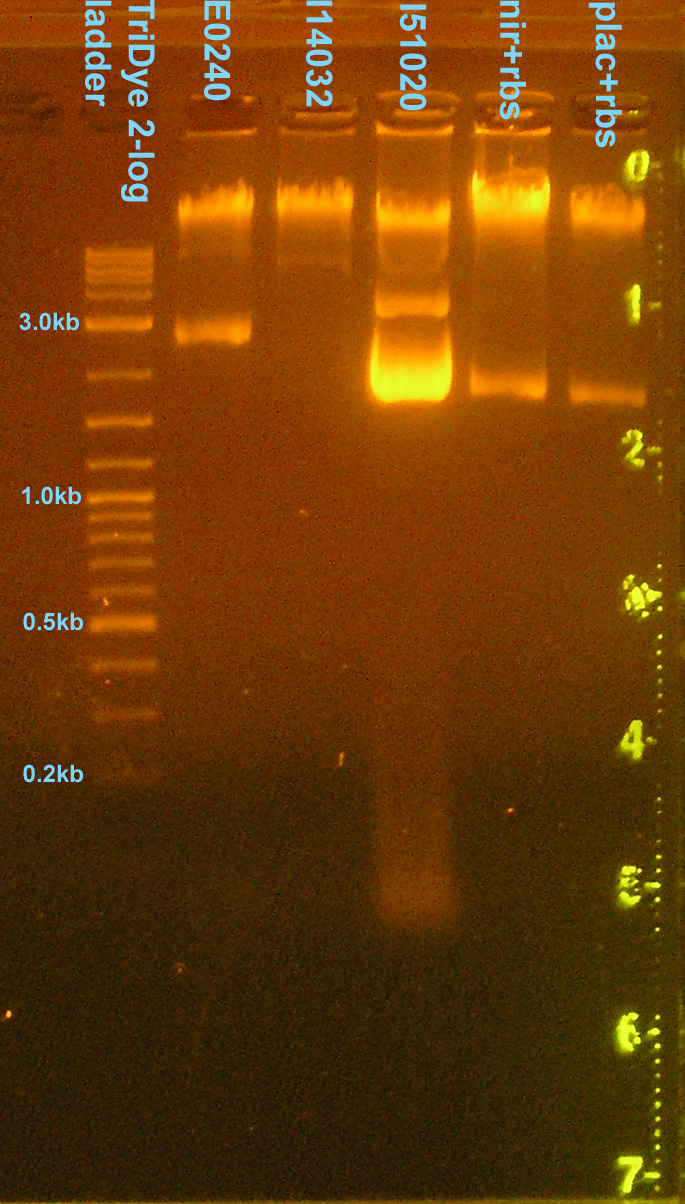
EtBr stained 0.8% agarose gel ran at 95V for 1 hour. Five microliters of plasmid were loaded into each well.
- Ran on 2.0% agarose gel to verify plasmids
- DNA didn't run. Agarose concentration too high. Redid on 0.8% gel.
- Genomic DNA up top?
- Clean prep (no RNA)!
- Only E0240 verified. All other bands wrong size (circular/supercoiled?)
- Checked DNA concentrations via nanodrop spectrometer
| Plasmid
| DNA concentration
| 260/280
| 260/230
|
| E0240
| 757.7 ng/μl
| 2.06
| 1.49
|
| I14032 (2005 distribution)
| 541.4 ng/μl
| 2.01
| 1.27
|
| I51020
| 2775.6 ng/μl
| 1.97
| 1.77
|
| nir+rbs
| 566.8 ng/μl
| 1.83
| 1.10
|
| plac+rbs
| 344.0 ng/μl
| 1.95
| 1.28
|
Made 1000x Amp,100 stock solution
- Grace
Reinoculated for cryostocking
- Grace
- I14032 from 2005 and 2008 distributions
Construction of GFP device
- Grace
- Extracted nir+rbs, plac+rbs, GFP, GFPf from gel ran yesterday
- B0015 could not be extracted because fragment was not visible under short wave UV
- Digestion was done for 3A assembly rather than rear ligation (oops). Redid RE digest.
- Checked DNA concentrations via nanodrop spectrometer
| Part
| DNA concentration
|
| nir+rbs
| 4.8 ng/μl
|
| plac+rbs
| 3.6 ng/μl
|
| GFP
| 4.7 ng/μl
|
| GFPfusion
| 6.4 ng/μl
|
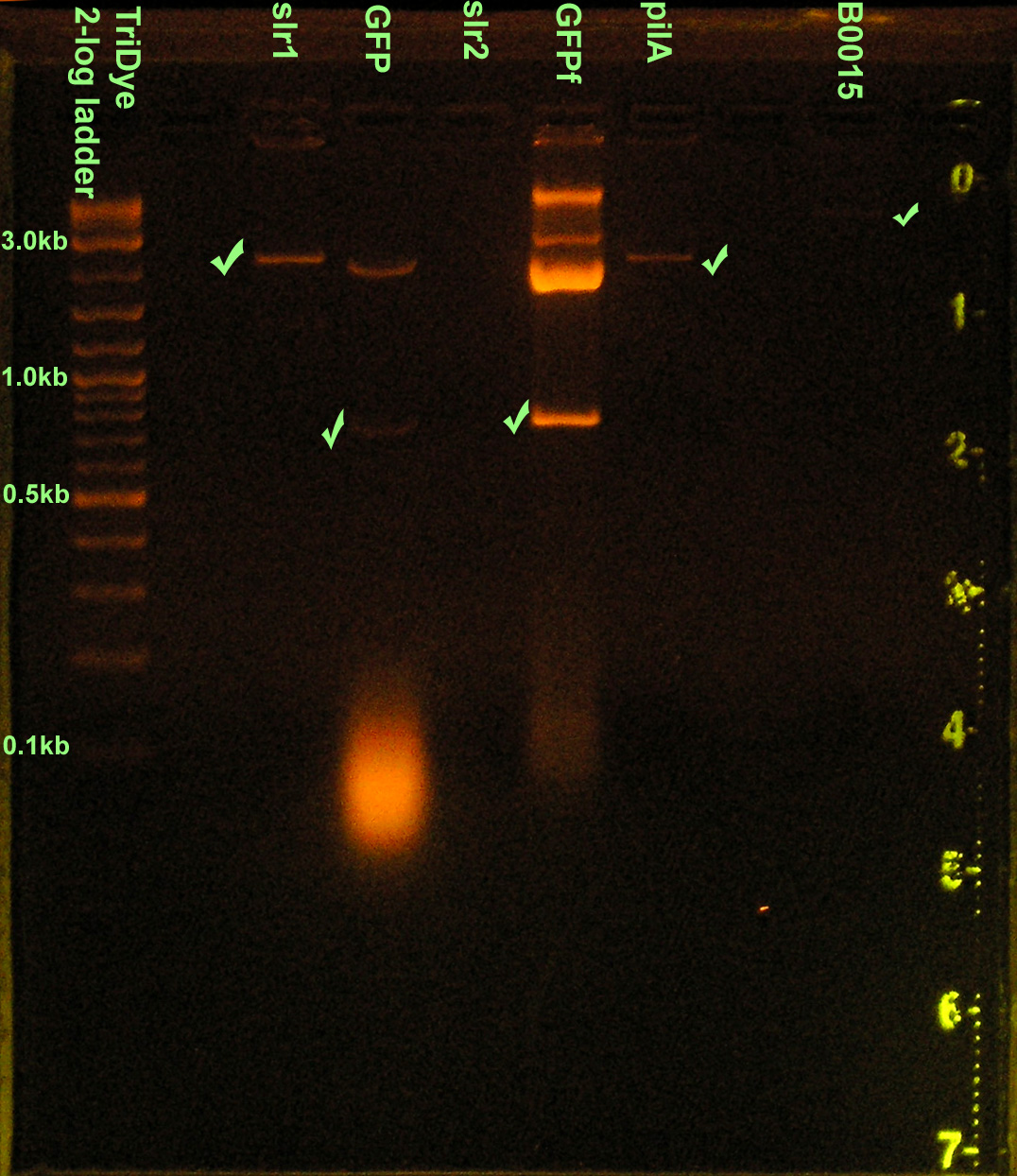
EtBr stained 2% agarose gel ran at 72V for 1.5 hours. Thirty microliters of the RE digest reactions were loaded into each well.
- Restriction digested in 30 μl reactions:
- B0015 with XbaI then EcoRI
- GFP and GFPf with EcoRI and SpeI
- slr1, slr2, pilA with SpeI and PstI
- Ran new RE digests EtBr stained 2% agarose gel at 72V for 1.5 hours
- Extracted parts from gel and determined DNA concentrations
| Part
| DNA concentration
|
| slr1
| 2.6 ng/μl
|
| pilA
| 1.1 ng/μl
|
| GFP
| 0.4 ng/μl
|
| GFPf
| 11.3 ng/μl
|
| B0015
| 1.9 ng/μl
|
- Ligated for 1 hour using Quick T4 DNA Ligase and Quick Ligase buffer:
- 8 μl GFP + 0.5 μl B0015
- 4 μl GFPf + 4 μl B0015
- 2 μl GFPf + 1.5 μl slr1
- 2 μl GFPf + 3.5 μl pilA
- Transformed 7 μl ligation reaction into DB3.1 cells
- RE digest overnight of 22 μl pSB1A2 with EcoRI and PstI for 3A assembly
Testing restriction enzymes in the lab's -20C freezer
- Grace
- Digested pRL1383a with BamHI (should result in a single linear fragment)
- Digested pRL1383a with HindIII (should result in a single linear fragment)
- Digested plasmid preps (E0240, I14032, I51020, nir+rbs, plac+rbs) with NotI (should result in two fragments -- vector and insert)
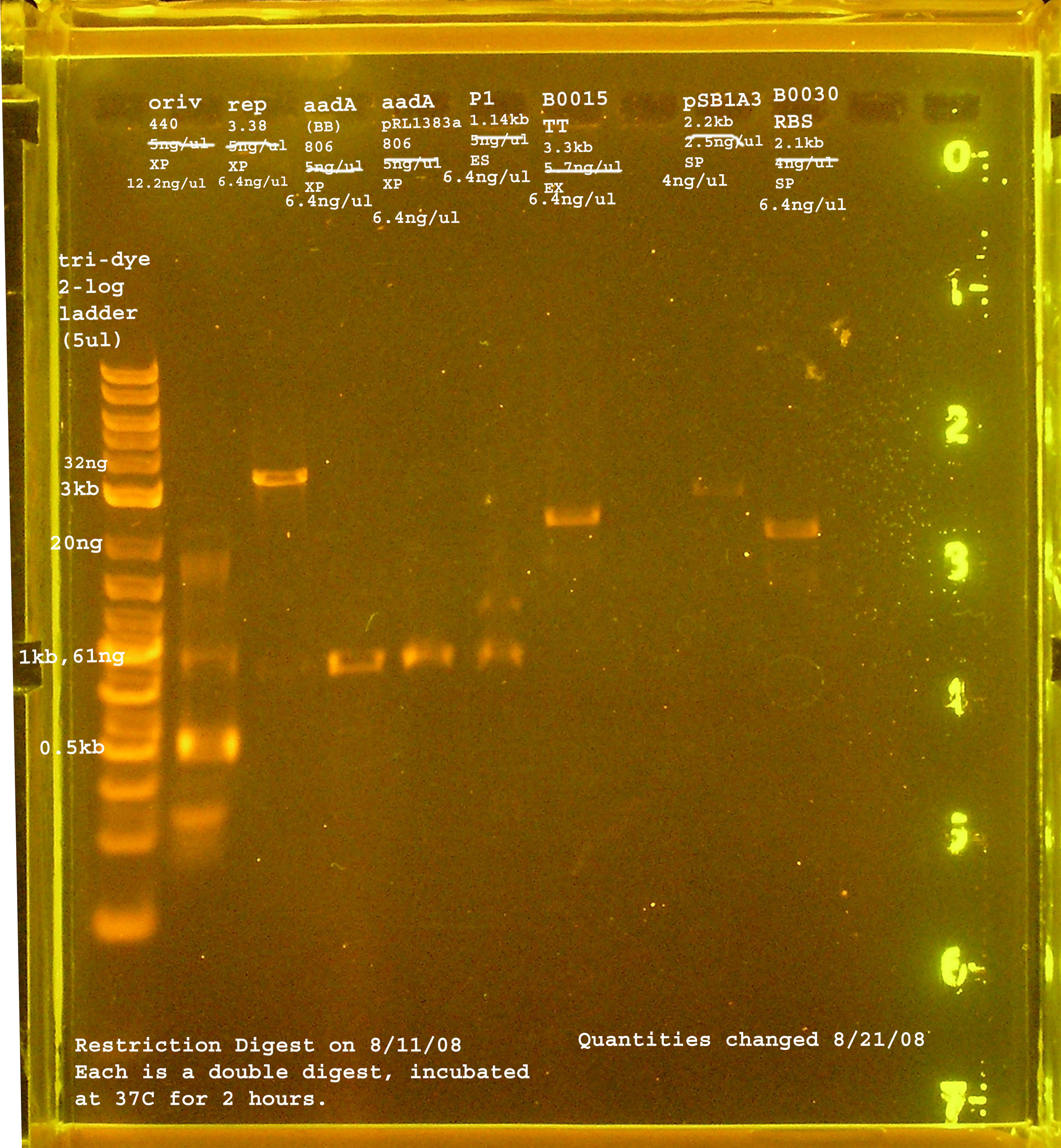
Restriction digest after 2 hours.

Ligation reaction after 2 hours.
- Margaret
- restriction digest of rep, oriV, aada(BB), aada(pRL1383a), P1 lytic region, pSB1A3, B0030, B0015
- Ligation: rep+B0030, oriV+pSB1A3, aadA(BB)+B0030, aadA(pRL1383a)+B0030, P1 lytic + B0015, pSB1A3 to itself (-) control
- Transformation into DH5-a (batch 3)
Started Culture for plasmid prep & cryostocks
- to be completed 8/12
- B0015, pSB3K3, oriT(cryostock & plasmid prep), B0030, I14032, E0040, J33207
Discussion
-
- According to the Endy lab, ligation reactions should have <100ng DNA per reaction for maximum efficiency
- ~10ng vector should be used in ligation reactions (6:1 ratio of insert to vector)
Quote of the Day
History is the only laboratory we have in which to test the consequences of thought. - Étienne Gilson
[http://manoa.hawaii.edu/  ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 ][http://manoa.hawaii.edu/ovcrge/
][http://manoa.hawaii.edu/ovcrge/  ][http://www.ctahr.hawaii.edu
][http://www.ctahr.hawaii.edu  ]
]
 "
"



