Team:Chiba/Calendar-Home/1 September 2008
From 2008.igem.org
31 August 2008 <|> 2 September 2008
Contents |
Laboratory work
Team:Input
- Ptet+RBS+cIの、Mini Prep産物の濃度チェック。
- -Ptet-cI-pMB1-Amp-の機能チェック。
- -Ptet-cI-pMB1-Amp-,-PcI-GFP-p15A-Cm-をDouble Transformation(BWΔflic)
- 結果-->GFPが発現していた。-->-PcI-GFP-p15A-Cm-が機能していないのでは?
- ターミネーターをつける、pSB1A3にのせかえる。
- -Ptet-cI-pMB1-Amp-のDigestion Check
混ぜ表
| Double | Single | |
| dH2O | 1 | 2 |
| XbaI | 1 | 1 |
| SpeI | 1 | - |
| BSA(×10) | 1 | 1 |
| NEB(×10) | 1 | 1 |
| DNA | 5 | 5 |
| TOTAL | 10 | 10 |
UV照射テスト
- -Ptrc-LuxR-Plux-cI-colE1-Amp-,-PcI-GFP-p15a-Cm-の2plasmid(BW)
- グリストをつついて2ml培養(UV⊕用と、UV⊖用の2本)
- 37℃,12h後UV測定(UV⊕:5.21,UV⊖:5.38)
- 小さなプレートにLB培地ごと流してUV照射開始。(UVは254nm,UVまでの距離は7.5cm,UV⊖のものは暗所に置いておく。)
- 乾かないようにポリエチレン製サランラップで蓋をした。
- UV⊕はUV照射後0min,10min,30min,1h,2h,4h,6h,8に、UV⊖は0h,1h,4h,8hによく攪拌してから、20μl採取。それぞれを
- 104,105希釈し、20μlをプレートに撒いた。
- 37℃,12h後にコロニーを数える。
コロニー数
| UV+ 104 | UV+ 105 | UV-104 | UV-105 | |
| 0min | 423 | 74 | 271 | 156 |
| 10min | 301 | 51 | - | - |
| 30min | 139 | 7 | - | - |
| 1h | 89 | 5 | 1040 | 54 |
| 2h | 5 | 1 | - | - |
| 4h | 2 | 0 | 254 | 50 |
| 6h | 0 | 0 | - | - |
| 8h | 1 | 0 | 1155 | 106 |
Team:Communication
- (31/8)--->Gel Check

|
|
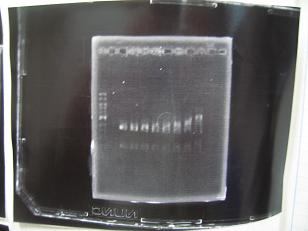
|
|
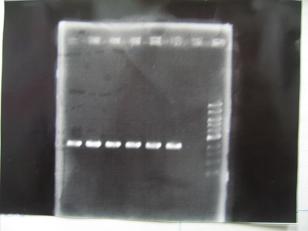
|
|
- --->Gel extract
- --->zymo
- insert-1(I9026) -> 7μL
- insert-2(I9030) -> 7μL
- insert-3(S03154) -> 7μL
- vector-4(R0010) -> 15μL
- --->SAP
- vector-4(R0010)
- --->Zymo
- vector-4(R0010) -> 20μL
- --->Gel Check

|
|
- --->Ligation
| Sample No. | (1) | (2) | (3) | (4) | (5) |
| insert-1(I9026) | 3 | - | 3 | - | - |
| insert-2(I9030) | - | 3 | - | 3 | - |
| vector-4(R0010) | 3 | 3 | - | - | 3 |
| ligase | 1 | 1 | 1 | 1 | 1 |
| Buffer | 1 | 1 | 1 | 1 | 1 |
| dH2O | 2 | 2 | 5 | 5 | 5 |
| TOTAL | 10 | 10 | 10 | 10 | 10 |
- --->Transformation
- Competent cells : XL10GOLD 30μL
- Transformed the following and grew on new ampicillin plates.
- [http://partsregistry.org/Part:BBa_K084009 BBa_K084009(Plac+RBS+RhlI+LVA, Amp)] -> 628 colonies
- [http://partsregistry.org/Part:BBa_K084010 BBa_K084010(Plac+RBS+CinI+LVA, Amp)] -> 500 colonies
- insert-1(RBS+RhlI+LVA) -> 9 colonies
- insert-2(RBS+CinI+LVA) -> No colonies on the plate
- vector-4(Plac, Amp) -> 186 colonies
- --->(2/9) Colony PCR
- Competent cells : JW1908 40μL
- Transformed the following and grew on new ampicillin plates.
- [http://partsregistry.org/Part:BBa_K084007 BBa_K084007(Plac+RBS+LasI)]
- [http://partsregistry.org/Part:BBa_K084008 BBa_K084008(Plac+RBS+RhlI)]
- [http://partsregistry.org/Part:BBa_K084010 BBa_T9002(Ptet+RBS+LuxR+GFP)]
- --->(2/9)Liquid Culture
Team:Output
- vector:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010] insert:[http://partsregistry.org/Part:BBa_J52008 BBa_J52008]①
- negative control:[http://partsregistry.org/Part:BBa_R0010 BBa_R0010]②
| Sample No. | ① | ② |
| vector | 2 | 2 |
| insert | 3 | 0 |
| Ligase Buffer | 2 | 2 |
| Ligase | 1 | 1 |
| dH2O | 3 | 5 |
| TOTAL | 10 | 10 |
-->R/T 2hour
- [http://partsregistry.org/Part:BBa_R0079 BBa_R0079](Las promoter)
- [http://partsregistry.org/Part:BBa_R0071 BBa_R0071](RhlR promoter)
- [http://partsregistry.org/Part:BBa_R0077 BBa_R0077](cinR promoter+RBS)
- [http://partsregistry.org/Part:BBa_R0078 BBa_R0078](cinR promoter)
- [http://partsregistry.org/Part:BBa_R0062 BBa_R0062](Lux promoter)
 "
"