Team:Montreal/Notebook
From 2008.igem.org
| Home | The Team | The Project | Parts Submitted to the Registry | Modeling | Notebook |
|---|
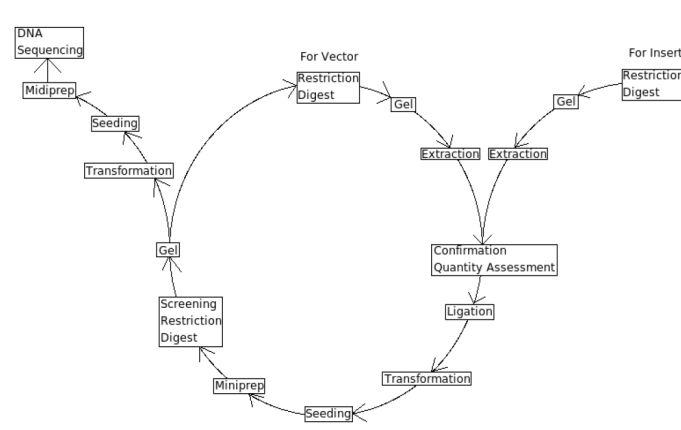
|
Lab Protocols
Key Elements of the Central Dogma of cloning
5. Ligation
8. DNA Extraction: Mini-, Midi-, Maxiprep
Lab Progress
May 2008 | June 2008 | July 2008
June 2nd, 2008:
- Growth of I-brick on culture
- Midi-prep of both I and J brick followed by gel
- Gel indicates no presence of DNA, will be confirmed by spectrophotometric assay
June 3rd, 2008:
- Seeding of 5mL cultures of both I and J brick
- Identity of colonies on I brick plates is suspect, must ensure that eventual DNA gel confirms exact restriction digest
June 4th, 2008:
- Midiprep was done on the I and the J-brick. Once the isopropanol was added, the J-brick midiprep looked clear (no DNA was eluted). DNA gel needs to be done to confirm presence of DNA in both cases.
June 9th, 2008:
- Seeded J and I-brick re-seeded for Maxi-Prep
- Gel failed to confirm presence of previously collected DNA samples of J and I-brick, will be repeated following Maxi-Prep.
June 10th, 2008:
- Since no growth was observed in the I-brick culture, the I brick was re-seeded.
- The J-brick was diluted in 500-ml of LB broth(for a Maxiprep to be done the next day).
June 11th, 2008:
- Maxiprep of the J-brick.
- Restriction digest of J-brick.
- Dilution of I-brick in 500-ml of LB broth.
June 12th, 2008:
- Maxiprep of the I-brick.
- Restriction digest and gel of June 11th and June 12th I-brick and J-brick DNA using EcoR1. Bands revealed at roughly 4000bp and 2500bp for I-brick (expected 2652bp and 3939bp). J brick single band that was not informative, new digestion to be completed tomorrow.
June 16th, 2008:
- I-brick was seeded and diluted over last two days, but there was insufficient growth so it will be left to grow one more day before performing another midi-prep. This is to compliment the already successful Maxi-Prep that gave low concentrations of DNA.
- Another gel was performed of previous J-brick preps that confirmed the absence of the desired plasmid, no DNA was detected when digested with EcoR1.
- J-brick was re-transformed into TOP10 chemically competent cells and then plated on Amp+ plates.<ul/></p>
 "
"